Electrons in the Atom
... 3. What is the energy released when a hydrogen electron moves from n=6 to n=2? 4. What is the difference between ground state and excited state? How do electrons move between these two states? 5. What does it mean for an atom to become an ion? How does the charge relate to the change in electrons? ...
... 3. What is the energy released when a hydrogen electron moves from n=6 to n=2? 4. What is the difference between ground state and excited state? How do electrons move between these two states? 5. What does it mean for an atom to become an ion? How does the charge relate to the change in electrons? ...
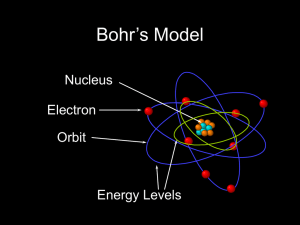
Rutherford–Bohr model
... The Rutherford–Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of ...
... The Rutherford–Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of ...
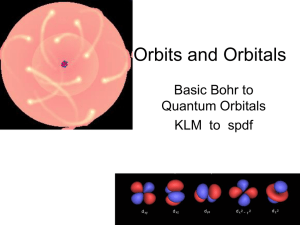
Orbits and Orbitals
... More rules • No orbital can have more than 2 e- in it. (one spin up, one spin down) • Orbitals are half filled (with spins in the same direction) before they are doubly filled. • Orbitals are filled from lowest energy to highest energy. ...
... More rules • No orbital can have more than 2 e- in it. (one spin up, one spin down) • Orbitals are half filled (with spins in the same direction) before they are doubly filled. • Orbitals are filled from lowest energy to highest energy. ...
Chapter 2 Part 1 ppt
... corresponding to an atomic orbital • The conditions for a physically realistic solution: -One value for electron density/point -Continuous (does not change abruptly) -Must approach zero as r approaches infinity ...
... corresponding to an atomic orbital • The conditions for a physically realistic solution: -One value for electron density/point -Continuous (does not change abruptly) -Must approach zero as r approaches infinity ...
Pauli Exclusion Principle Quiz
... Pauli Exclusion Principle Quiz 1. The location of any electron in an atom can be described by ____ unique quantum numbers. ...
... Pauli Exclusion Principle Quiz 1. The location of any electron in an atom can be described by ____ unique quantum numbers. ...
Electrons #1
... We can determine the position of an e- from the nucleus through 4 Quantum Numbers: 1. Principle Number (Energy of e-) 2. Angular Momentum Number (Shape of Orbital) 3. Magnetic Number (Orientation/Position of Orbital) 4. Spin Number (Spin on e- ; +/-) ...
... We can determine the position of an e- from the nucleus through 4 Quantum Numbers: 1. Principle Number (Energy of e-) 2. Angular Momentum Number (Shape of Orbital) 3. Magnetic Number (Orientation/Position of Orbital) 4. Spin Number (Spin on e- ; +/-) ...
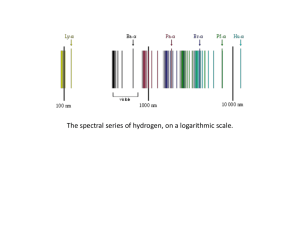
Atomic spectra and the Bohr atom
... of same n by giving them different shapes; any integer value from 0 to n-1; orbitals of same n but different l are in different sub-shells: s p d f g ...
... of same n by giving them different shapes; any integer value from 0 to n-1; orbitals of same n but different l are in different sub-shells: s p d f g ...
Chapter 13 – Electrons in Atoms
... Atoms/elements emit light when the electrons are excited (first absorb then emit energy in the form of light) at specific frequencies. ...
... Atoms/elements emit light when the electrons are excited (first absorb then emit energy in the form of light) at specific frequencies. ...
Quantum numbers
... • Carbon: (1s2) 2s2, 2p2; Sulfur: (…), 3s2, 3p4 • Homework: write down the electron configurations of N, O, Cl why do halogens (X) form X2 in the gas phase? why do the alkali metals (Li, Na, ….) do so too? ...
... • Carbon: (1s2) 2s2, 2p2; Sulfur: (…), 3s2, 3p4 • Homework: write down the electron configurations of N, O, Cl why do halogens (X) form X2 in the gas phase? why do the alkali metals (Li, Na, ….) do so too? ...
Ch 11 WS Orbitals and Electron Arrangement
... the probability of finding an electron in a certain position. ...
... the probability of finding an electron in a certain position. ...
Chemical Building Blocks
... A rubidium isotope has 50 neutrons. What is its mass no.? How many neutrons does 90Mo have? How many neutrons are in bromine-81? Which of the following isotopes are of the same element? Name the isotopes. ...
... A rubidium isotope has 50 neutrons. What is its mass no.? How many neutrons does 90Mo have? How many neutrons are in bromine-81? Which of the following isotopes are of the same element? Name the isotopes. ...
Writing Electron Configuration
... Pauli Exclusion Principle • No two electrons may have the same 4 quantum numbers. • This means: • Maximum of 2 electrons occupy a single atomic orbital • The 2 electrons must have opposite spins, represented by up/down arrows. ...
... Pauli Exclusion Principle • No two electrons may have the same 4 quantum numbers. • This means: • Maximum of 2 electrons occupy a single atomic orbital • The 2 electrons must have opposite spins, represented by up/down arrows. ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.