* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bands - abuad lms
Survey
Document related concepts
Transcript
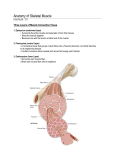
EBUNILO KRYSTAL CHIAMAKA 14/MHS02/021 NURSING SCIENCE 200 LEVEL ANA 203 ASSIGNMENT LINES AND BANDS OF MUSCLE TISSUE A sarcomere is the basic unit of striated muscle tissue. Skeletal muscles are composed of tubular muscle cells (myocytes called muscle fibers) which are formed in a process known as myogenesis. Muscle fibers are composed of tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long, fibrous tail and a globular head, which binds to actin. The myosin head also binds to ATP, which is the source of energy for muscle movement. Myosin can only bind to actin when the binding sites on actin are exposed by calcium ions. Actin molecules are bound to the Z line, which forms the borders of the sarcomere. Other bands appear when the sarcomere is relaxed.]A muscle fiber from a biceps muscle may contain 100,000 sarcomeres. The myofibrils of smooth muscle cells are not arranged into sarcomeres. Bands Muscle contraction based on sliding filament hypothesis The sarcomeres are what give skeletal and cardiac muscles their striated appearance. A sarcomere is defined as the segment between two neighbouring Z-lines (or Z-discs, or Z bodies). In electron micrographs of cross-striated muscle, the Z-line (from the German "Zwischenscheibe", the disc in between the I bands) appears as a series of dark lines. Surrounding the Z-line is the region of the I-band (for isotropic). I-band is the zone of thin filaments that is not superimposed by thick filaments. Following the I-band is the A-band (for anisotropic). Named for their properties under a polarizing microscope. An A-band contains the entire length of a single thick filament. Within the A-band is a paler region called the H-zone (from the German "heller", brighter). Named for their lighter appearance under a polarization microscope. H-band is the zone of the thick filaments that is not superimposed by the thin filaments. Inside the H-zone is a thin M-line (from the German "Mittelscheibe", the disc in the middle of the sarcomere) formed of cross-connecting elements of the cytoskeleton. The relationship between the proteins and the regions of the sarcomere are as follows: Actin filaments, the thin filaments, are the major component of the I-band and extend into the A-band. Myosin filaments, the thick filaments, are bipolar and extend throughout the A-band. They are cross-linked at the centre by the M-band. The giant protein titin (connectin) extends from the Z-line of the sarcomere, where it binds to the thick filament (myosin) system, to the M-band, where it is thought to interact with the thick filaments. Titin (and its splice isoforms) is the biggest single highly elasticated protein found in nature. It provides binding sites for numerous proteins and is thought to play an important role as sarcomeric ruler and as blueprint for the assembly of the sarcomere. Another giant protein, nebulin, is hypothesised to extend along the thin filaments and the entire I-Band. Similar to titin, it is thought to act as a molecular ruler along for thin filament assembly. Several proteins important for the stability of the sarcomeric structure are found in the Zline as well as in the M-band of the sarcomere. Actin filaments and titin molecules are cross-linked in the Z-disc via the Z-line protein alpha-actinin. The M-band proteins myomesin as well as C-protein crosslink the thick filament system (myosins) and the M-band part of titin (the elastic filaments). The interaction between actin and myosin filaments in the A-band of the sarcomere is responsible for the muscle contraction (sliding filament model). Contraction Upon muscle contraction, the A-bands do not change their length (1.85 micrometer in mammalian skeletal muscle), whereas the I-bands and the H-zone shorten. This causes the Z lines to come closer together. The protein tropomyosin covers the myosin binding sites of the actin molecules in the muscle cell. To allow the muscle cell to contract, tropomyosin must be moved to uncover the binding sites on the actin. Calcium ions bind with troponin-C molecules (which are dispersed throughout the tropomyosin protein) and alter the structure of the tropomyosin, forcing it to reveal the crossbridge binding site on the actin. The concentration of calcium within muscle cells is controlled by the sarcoplasmic reticulum, a unique form of endoplasmic reticulum in the sarcoplasm. Muscle contraction ends when calcium ions are pumped back into the sarcoplasmic reticulum, allowing the contractile apparatus and, thus, muscle cell to relax. During stimulation of the muscle cell, the motor neuron releases the neurotransmitter acetylcholine, which travels across the neuromuscular junction (the synapse between the terminal bouton of the neuron and the muscle cell). Acetylcholine binds to a post-synaptic nicotinic acetylcholine receptor. A change in the receptor conformation allows an influx of sodium ions and initiation of a post-synaptic action potential. The action potential then travels along T (transverse) tubules until it reaches the sarcoplasmic reticulum. Here, the depolarized membrane activates voltage-gated L-type calcium channels, present in the plasma membrane. The L-type calcium channels are in close association with ryanodine receptors present on the sarcoplasmic reticulum. The inward flow of calcium from the L-type calcium channels activate ryanodine receptors to release calcium ions from the sarcoplasmic reticulum. This mechanism is called calcium-induced calcium release (CICR). It is not understood whether the physical opening of the L-type calcium channels or the presence of calcium causes the ryanodine receptors to open. The outflow of calcium allows the myosin heads access to the actin cross-bridge binding sites, permitting muscle contraction. ] Rest At rest, the myosin head is bound to an ATP molecule in a low-energy configuration and is unable to access the cross-bridge binding sites on the actin. However, the myosin head can hydrolyze ATP into adenosine diphosphate (ADP) and an inorganic phosphate ion. A portion of the energy released in this reaction changes the shape of the myosin head and promotes it to a high-energy configuration. Through the process of binding to the actin, the myosin head releases ADP and an inorganic phosphate ion, changing its configuration back to one of low energy. The myosin remains attached to actin in a state known as rigor, until a new ATP binds the myosin head. This binding of ATP to myosin releases the actin by cross-bridge dissociation. The ATPassociated myosin is ready for another cycle, beginning with hydrolysis of the ATP. The A-band is visible as dark transverse lines across myofibers; the I-band is visible as lightly staining transverse lines, and the Z-line is visible as dark lines separating sarcomeres at the lightmicroscope level.