* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CNM
Nucleic acid analogue wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosynthesis wikipedia , lookup
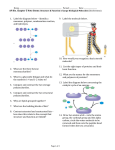
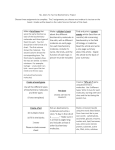
CNM Nutritional Biochemistry Workbook CNM www.naturopathy-uk.com The College of Naturopathic Medicine Nutritional Biochemistry Revision Workbook - Please note there is no answer booklet, this is to assist study only Prepared by Holly Taylor © Copyright 2008/9 CNM :V1: Issued 08/2008 Page 1 of 42 CNM Nutritional Biochemistry Workbook Section 1 - Chemistry Definitions – Provide definitions and examples for the following Chemical entity Definition Example a) Matter b) Atom c) Element d) Ion e) Molecule f) Compound g) Hydrophilic molecule h) Hydrophobic molecule Which four elements are the major constituents of the human body? 1 2 3 4 Page 2 of 42 CNM Nutritional Biochemistry Worksheet Atomic structure - Label the follow picture of an atom Name: Name: Charge: Name: Charge: Name: Charge: The periodic table – Fill in the gaps The periodic table is a list of all of the known …………………………………arranged into periods that show us which……………………. have similar ……………………….and physical properties. Label the following picture The number that is assigned to each element correlates to the amount of................and………………in each element Page 3 of 42 CNM Nutritional Biochemistry Worksheet Give the chemical symbol for the following elements Hydrogen = Oxygen = Sodium= Carbon = Sulfur = Magnesium= Nitrogen = Potassium = Iron = Counting subatomic particles - Complete the following table Element Number of Number of Number of protons neutrons electrons What is an isotope? Give an example of an element with 2 abundant stable isotopes Page 4 of 42 CNM Nutritional Biochemistry Worksheet Electron shells - Fill in the gaps The bonding properties of an element are dependant on the amount of ……………it has it its outer shell. Atoms are always trying to ………………their outer shells. Some elements do not easily react as they have their outer shell……………… to the perfect number. We call these substances………………. The……………………in an elements outer shell are called the ……………… ………………. Oxidation and reduction – Complete the following Oxidation is ……………………………………………………………………………………………………………………… Reduction is ……………………………………………………………………………………………………………………. When oxidation and reduction happen together is it known as a ……………… reaction. Why is oxidation important in the human body? What is a free radical and what kind of damage do they cause? Suggest 3 things that contribute to our free radical load 1 2 3 Page 5 of 42 CNM Nutritional Biochemistry Worksheet What is an antioxidant and how does it work? Bonding The 2 types of bonding are (Given the proper term and a description of what happens to the electron) a) …………………………………………………………………………………………. b) ………………………………………………………………………………………….. Fill in the gaps …………………………bonds are formed when one element has more electron pulling power than the other element in the bond. This results in an ……………………distribution of charge where one end of the molecule is ………………….. other end is …………………………… ………………………. Name the four most electronegative elements 1 2 3 4 Page 6 of 42 ………………….. and the CNM Nutritional Biochemistry Worksheet Draw a picture of a hydrogen bond and given an example of a substance in which you might find hydrogen bonding occurring Example………………………………………………. Fill in the gaps Van der Waals forces are an ………………..reaction that occurs in …….. molecules and atoms. It happens because electrons are……………….. At any one instant the electrons might find themselves towards one end of the molecule, making that end slightly …………….This leaves the other end temporarily short of electrons so it becomes slightly…………………… Explain the term dipole ……………………………………………………………………………………………… Please shade in the molecules below and add positive and negative charges to show how Van der Waals force help to hold a substance together Page 7 of 42 CNM Nutritional Biochemistry Worksheet Fill in the gaps Van der Waals force between molecules are much ……………..than the covalent bonds. Bigger molecules with more electrons form …………… Van der Waals forces. ………, ………… molecules can develop bigger temporary dipoles due to electron movement than ……………., …………. Ones. Straight molecules can also get closed to each other than bent molecules so the attraction forces are…………………… Electrolytes – Answer the following What is an electrolyte? Give 3 important biological functions of electrolytes 1 2 3 Acids and bases An acid is ……………………………………….……………………………………………… Example: ………………………………………………………………………………………. A base is ……………………………………………………………………………………….. Example: ………………………………………………………………………..……………… What scale is used to measure acidity and basicity? Page 8 of 42 CNM Nutritional Biochemistry Worksheet Where does water appear on this scale? Acid + Base => ……………………… + ……………………… Buffers – Complete the following Buffers are substances that maintain the ………………… concentration, to help the body maintain homeostasis. Some buffer bind ……………….. and other bind ………………………… Give an example of a buffer system used by the body ……………………………………………………………………………………………………………………………… Energy – Complete the following The law of conservation of energy states that………………………………………………………………. …………………………………………………………………………………………………………………………………………. Name 4 different types of energy 1 2 3 4 Page 9 of 42 CNM Nutritional Biochemistry Worksheet Changes of state Label the 3 boxes below with the names of the 3 main states of matter then draw a sketch of how closely the particles in each state are arranged. Underneath briefly describe the properties of each state in terms of shape volumes and particle speed Chemical reactions – Fill in the gaps A reaction occurs when …………………. are broken or created. The starting materials are known as……………………and the end materials are called………………….. All reaction involve a transfer of ………………….. For a reaction to occur the molecules must………………………with the correct ………………. The minimum ………………. At which a reaction will occur is called the energy of ……………… An endogonic reaction is ……………………………………………………………. An exergonic reaction ……………………………………………………………….. Give 3 physical properties a chemical reaction can be affected by a) b) c) Page 10 of 42 CNM Nutritional Biochemistry Worksheet Explain what a catalyst does……………………………………………………………………………………… …………………………………………………………………………………………………………………………..…….... Explain what an inhibitor does………………………………………………………………………………….. …………………………………………………………………………………………………………………………… ……… Complete the following table Type of reaction Schematic Endo- or exer- gonic? Anabolic Synthesis/Building reaction AB => A + B Exchange reaction Can be either A reversible reaction exists in a state of …………………………….…….. where there is always some reactants and some products present. When water is the medium that breaks down a molecule into smaller pieces it is called a ……………………………… reaction When water is formed as a waste product during an anabolic reaction, it is known as a …………………………. Page 11 of 42 …………………………… reaction CNM Nutritional Biochemistry Worksheet What is special about reversible reactions? Give a brief outline of Le Chateliers principle ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………….. Functional groups Place the name the following functional beside each diagram Outline the key properties of each group R-O-H R-S-H Page 12 of 42 CNM Nutritional Biochemistry Worksheet R Section 2 - Carbohydrates Give 3 examples of carbohydrates 1 2 3 What 3 elements are carbohydrates made up of? Complete this table Diagram Type of Number of carbohydrate units Examples Glucose 2 Polysaccharide Page 13 of 42 CNM Nutritional Biochemistry Worksheet Give 2 properties of a polysaccharide that make it different to the other two type of carbohydrate a)……………………………………………………………………………………… b)……………………………………………………………………………………… What kind of bonds are found between the sugar molecules of a carbohydrate? What kind of reaction is needed to join sugar molecules together? Explain the term isomer………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………... ………………………………………………………………………………………………………………………………………… Name the 2 different types of starch 1 2 What is glycogen and what is it used for? Page 14 of 42 CNM Nutritional Biochemistry Worksheet Fill in the gaps Cellulose is structural material of ……………………. It is a …………………… polymer. Human don’t have the …………… required to break these beta (1,4) glycosidic bonds, so cellulose is not digestible by humans and is often referred to as ……………. ………….. Some animals, particularly ………………. and …………………., can digest cellulose with the help of ……………………………………………………………………………………………………………………………. Suggest 3 functions of carbohydrate 1 2 3 Carbohydrates are progressively digested at 3 different sites in the body. Name the 3 sites and outline what happens there 1 2 3 What is the name of the main carbohydrate digesting enzyme? ........................... The glucose form carbohydrate digestion can be used in a number of processes. Name 3 of them 1 Page 15 of 42 CNM Nutritional Biochemistry Worksheet 2 3 Section 3 - Lipids Which 3 elements are lipids made up of? What is different about the elements and bonding in lipids compared to carbohydrates? …………………………………………………………………………………………………………………………………………. In what form are lipids transported around the body?............................................ Label the following diagram Bond = Fill in the gaps Triglycerides are formed by a ………………………. broken down by ………………………….. Page 16 of 42 …………………………. reaction and CNM Nutritional Biochemistry Worksheet Give 3 functions of triglycerides 1 2 3 Explain the difference between saturated, monounsaturated and polyunsaturated fats. ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………..…………………………………………... Give the omega name for the following type of fat …………………………………………………. Put 2 hydrogen atoms on the carbon double bonds below and label them cis and trans C=C ………………….. C=C ………………… Which type - cis or trans - is favoured and why? ………………………………………………………. Page 17 of 42 CNM Nutritional Biochemistry Worksheet ……………………………………………………………………………………………………………………………………….. Explain the term essential fatty acid and give one example from each of the 2 families An EFA is ………………………………………………………………………………………………………………. Example 1………………………………. Family = …………………………………. Example 2………………………………. Family = …………………………………. Name 5 functions of EFAs 1 2 3 4 5 Why should you never heat polyunsaturated fats and EFA’s? What is a lipoprotein? Which type of lipoprotein do we refer to as good cholesterol? Page 18 of 42 ............................ CNM Nutritional Biochemistry Worksheet Complete this table Type of lipoprotein Function Carry triglycerides from the intestines to the liver, muscles and adipose tissue VLDL LDL Collect cholesterol from body tissues and takes it back to the liver Label and name the molecule below. Where would you expect to find it in the body? Name: Found in: Page 19 of 42 CNM Nutritional Biochemistry Worksheet What is special property does this type of molecule have and what is the scientific term for this? i)…………………………………………………………………………………………… ii)…………………………………………………………………………………………... Give an example of a steroid and name the molecule from which steroids are made in the body. i)…………………………………………………………………………………………… ii)…………………………………………………………………………………………... Describe the role of the following in fat digestion Bile …………………………………………………………………………………………………………………………………… Pancreatic lipase……………………………………………………………………………………………………………… Small intestine………………………………………………………………………………………………………………… Page 20 of 42 CNM Nutritional Biochemistry Worksheet Section 4 - Proteins and amino acids Amino acids are the building blocks of protein. They are formed from the elements ……………………, ……………………., ……………………, and …………………. Some also contain ………………….. ……………… amino acids are found in human proteins Every amino acid has a ……………………………. group and an ……………………………. group. Each individual amino acids has a …………………… that determines the amino acid’s characteristics. This is usually labelled as ……….. Label this diagram Give the names of the four different categories of amino acid an a specific example for each one. 1………………………………………………….. e.g.…………………………………………….. 2………………………………………………….. e.g.…………………………………………….. Page 21 of 42 CNM Nutritional Biochemistry Worksheet 3…………………………………………………... e.g.……………………………………………… 4……………………………………………………. e.g.…………………………………………….. Explain the following terms and give an example of each Essential amino acid………………………………………………………………………………………………………. Example…………………………… Non-essential amino acid……………………………………………………………………………………………….. Example ………………………… Conditionally essential amino acid…………………………………………………………………………………. Example………………………….. Fill in the gaps Amino acids come in mirror images called …………………………………. These mirror images are either called the D or the L form. The human body only recognises the ……………form. Amino acids are joined together in ……………………………. reactions to make protein chains called ………………….. The bonds formed are called …………………. bonds Explain the following terms and give an example Dipeptide………………………………………………………………………………………………………………………… Example………………………………………………………. Tripeptide………………………………………………………………………………………………………………………… Page 22 of 42 CNM Nutritional Biochemistry Worksheet Example………………………………………………………. All free amino acids plus charged amino acids in peptide bonds can act as ………………………………… Where in a protein would you expect to find a polar amino acid and why? Explain what is meant by the term primary protein structure. ……………………………………………………………………………………………………………………………………… Give 2 examples of secondary structure you might find in a protein molecule i) ii) Explain what the tertiary structure of a protein is and why it is important ……………………………………………………………………………………………………………………………………… What is a disulfide bond and why is it important? Explain what the quaternary structure of a protein is ……………………………………………………………………………………………………………………………………… Explain denaturation ………………………………………………………………………………………………….. Page 23 of 42 CNM Nutritional Biochemistry Worksheet ……………………………………………………………………………………………………………………………………… What might cause a protein to misfold? Give 5 functions of protein in the human body 1 2 3 4 5 Describe the role of the following in protein digestion Stomach…………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… Pancreas…………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Small intestine ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… Page 24 of 42 CNM Nutritional Biochemistry Worksheet Summary - Macromolecules Complete this table Macromolecule Building blocks Type of bond Reaction Reaction formed by broken down by Protein Dehydration synthesis Carbohydrate Triglyceride Page 25 of 42 Monosaccharides Hydrolysis CNM Nutritional Biochemistry Worksheet Section 5 - Nucleic acids Name the 2 most common nucleic acids 1 2 Label the following diagram of a nucleotide Name of component ……………………………… 2 different types i)………………………. Name of component II)……………………… …………………………… Name of component ……………………………………… Type in DNA……………………… Type in RNA……………………… Name the 4 nucleotide used to make DNA 1 2 3 Page 26 of 42 CNM Nutritional Biochemistry Worksheet 4 What is different about the nucleotides in RNA? Explain the differences between the structure of DNA and RNA ……………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………… Fill in the complementary RNA base sequence for this strand of DNA RNA A G C T T G C G A DNA In base pairing a ………………………. always pairs with a …………………………… Base pair are held together by …………………………….. bonds. What are the 2 main functions of DNA? 1 2 What is the main function of RNA? Page 27 of 42 CNM Nutritional Biochemistry Worksheet Fill in the gaps The sequence of bases in DNA is called the ……………….. code. The code is what’s called a ………………….. code. The bases are read in sets of ……………… Each set is called a ……………. Each …………… corresponds to a specific ………………. ………………. or a start or stop at the end of a protein chain. Name the 2 stages of protein synthesis and briefly describe what happens. Stage 1 ………………………………………… What happens ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Stage 2 ………………………………………… What happens ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Page 28 of 42 CNM Nutritional Biochemistry Worksheet Section 6 - Enzymes What is an enzyme? ……………………………………………………………………………………………………… The molecules enzymes react with are called …………………………… Enzymes generally end in the suffix ………………… Many biological reactions are very………………….. Enzymes ……………… reactions by temporarily binding to one or more reactants, providing an alternative ……………. ……………., which has a lower ……………….. ……………….. Give 3 properties of enzymes 1 2 3 Explain how enzymes work using the lock and key analogy …………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. Page 29 of 42 CNM Nutritional Biochemistry Worksheet Some enzymes require…………………for activity, these can be metal ions or derivatives of vitamins. The protein part of the enzyme without its cofactors is known as the ………………………….. Explain how the following can affect enzymes Substrate concentration …………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. Temperature …………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. pH …………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. Draw a typical graph to show how the above parameters affect enzyme activity Substrate concentration Page 30 of 42 Temperature pH CNM Nutritional Biochemistry Worksheet Explain the following terms Inhibitor ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………..… Reversible inhibitor ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………..… Competitive inhibitor ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………..… Non-competitive inhibitor ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………..… Where might an inhibitor be used? Page 31 of 42 CNM Nutritional Biochemistry Worksheet Section 7 – Energy production ATP What does ATP stand for? ………………………………………………………………………………………… What are the 3 building blocks of ATP? 1 2 3 What is the main function of ATP? …………………………………………………………………………………………………………………………………………. ATP + H2O + ATPase => ……….. + …………… + ……………… What type of reaction is this? ………………………………….. Page 32 of 42 CNM Nutritional Biochemistry Worksheet This reaction can also go back the other way to store energy, catalysed by ………………………………… The energy is generally supplied by the process of ………………… …………………… The addition of a phosphate group is called ………………………….. Which mineral binds to ATP and aid the release the energy? Give 4 functions of ATP 1 2 3 4 Name the 2 processes by which ATP can be made and where in the cell they occur 1……………………………………… Occurs in ………………………. 2………………………………………. Occurs in ……………………….. NAD+ and FAD NAD+ and FAD are …………… carrying molecules. They carry high energy ………………………… NAD+ ………………………. substrates by stealing H-. In this process NAD+ is ……………….. and becomes ………………….. FAD can……………………… other molecules to become ………………. Page 33 of 42 CNM Nutritional Biochemistry Worksheet NAD+ is used in enzyme-catalysed …………………….. reactions e.g. ……………………………………………………………………………………………………… FAD is used in ……………… energy production and …………….. metabolism NAD+ can be synthesised from the amino acids ………………… or ………………………. It can also be obtained from vitamin …………… FAD is derived from ……………………………….. also known as vitamin …………….. Energy from carbohydrates What is the name of the process where glucose is oxidised to form ATP? What are the four steps of glucose metabolism? 1 2 3 4 Glycolysis The process of glycolysis turns …………………….. into 2 …………………. molecules. There is an input of ………. ATP molecules and an output of ………. ATP molecules. Net gain of ATP is ……….. In …………………….. conditions, glycolysis is the primary energy production pathway. When no oxygen is present …………………….. stays in the ………………. And is degraded to waste products In yeast pyruvate is converted to ……………………….. In muscle cells pyruvate is converted to ……………… ………………. This occurs because in anaerobic conditions ………………….. cannot be recycled in the electron transport Page 34 of 42 CNM Nutritional Biochemistry Worksheet chain. To allow energy production to continue ……………… gives its H- to …………………. This ……………… reaction turn pyruvate into ……………….. ………………. Coenzyme A Coenzyme A is a ……………….. molecule which is a form of ……………… …...... also known as vitamin ……….. It carries energy as an easily transferable high-energy bond with an……………. group. Acetyl CoA formation is catalysed by ……………….. carbon as ……………….. ……………………. Pyruvate loses a ……………… then joins to Coenzyme A Kreb cycle Where does the Kreb cycle occur? What happens to the 2 carbons from pyruvate during the Kreb cycle? Where is the energy generated by the Kreb cycle transferred to? How many turns of the Kreb cycle are there for each glucose molecule? The electron transport chain The electron carriers………….. and ……………… transfer their electrons to the electron transport chain. This is a chain of ………………….. electron carrier embedded in the ……………………………… ………………………………. The electrons are passed from one electron acceptor to the next and successively give up …………………….. The energy lost from the electrons is used to ………………………………………………………….. This creates an ……………………………. where there are more ………ions on one side of the membrane than the other. Page 35 of 42 CNM Nutritional Biochemistry Worksheet At the end of the chain the electron combine with …………… and …………… to form water. The …… ……………….. is used to drive phosphorylation of ADP to produce ……….. Name 4 nutrients needed for the electron transport chain 1 2 3 4 The energy released from NADH can be used to make ………. ATP The energy released form FADH2 can be used to make ………. ATP Page 36 of 42 CNM Nutritional Biochemistry Worksheet Summary – Energy production – Complete this diagram Glucose Overall glycolysis makes: 1) Glycolysis If no oxygen is present i)……………. ii)…………… iii)…………… If oxygen is present 2) MITOCHONDRIA Acetyl CoA One turn of the cycle produces i)…………… CO2 3) ii)…………. iii)…………. CO2 ii) iii) 4) Page 37 of 42 Water + ATP CNM Nutritional Biochemistry Worksheet Overall the reaction is C6H12O6 + ……O2 +…..ADP’s+ ……P……CO2 +….H2O +…..ATP Which group of vitamins is essential for energy production? Energy from fats In the absence of sufficient………………………… fatty acids can be used for energy production. Fat metabolism is stimulated by…………………. and inhibited by ………………… ………………….. splits fatty acids from adipose tissue. Fatty acids are then carried by ……………… ………………… to the liver where …………….. occurs. What is the name of the process used to degrade fatty acids and where does it occur? Name……………………………….. Occurs in……………………………… Explain how the glycerol component of a fat can be used to produce energy ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………….. Which nutrient helps transport fatty acids into the mitochondria? Page 38 of 42 CNM Nutritional Biochemistry Worksheet What are the 4 steps of beta-oxidation 1 2 3 4 How many carbons are cleaved off a fatty acid by each cycle of beta oxidation and which molecule do they become? Number of carbons = Released as……………………………………. Name the 3 products of beta-oxidation and explain which energy production system they are used in 1………………………………. Used in……………………………….. 2..……………………………. Used in……………………………….. 3………………………………. Used in……………………………….. The amount of energy release form a fat depends on what? Which key organ cannot use fatty acids for energy? When are ketone bodies produced? Page 39 of 42 CNM Nutritional Biochemistry Worksheet Where are ketone bodies made? Ketone bodies are made from acetyl CoA. This can come from 2 different dietary sources. What are these sources? 1 2 Give the names of the 3 ketone bodies 1 2 3 Explain the following terms Ketogenesis ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Ketosis ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Ketoacidosis ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Page 40 of 42 CNM Nutritional Biochemistry Worksheet How would you identify a person with ketoacidosis? Energy from protein The carbon skeletons of all amino acids are broken down into metabolites that can enter the ……………… …………………. From here they can either be completely ………………….. into CO2 , or diverted into …………………………. or …………………….. What has to happen to all amino acids before they can be used for energy production? Name 2 important vitamin cofactors for the degradation of amino acids 1 2 What waste product is produced during amino acid degradation and where does it end up? Waste product……………………………………… End up …………………………….. or ……………………………… Gluconeogenesis Explain the term gluconeogenesis ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. Page 41 of 42 CNM Nutritional Biochemistry Worksheet Suggest 3 molecules that could be used for gluconeogenesis 1 2 3 Where does gluconeogenesis occur? When does gluconeogenesis occur? What is needed for gluconeogenesis to take place? Which other pathway is gluconeogenesis very similar to? Name a nutrient needed for gluconeogenesis Energy from food What are the four major energy sources for the body 1 3 2 4 Which are the only 2 energy sources that can be used by the brain? THE END! Page 42 of 42