* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The only sure evidence that a chemical reaction has occured is
Fine chemical wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Drug discovery wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Biochemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Asymmetric induction wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical industry wikipedia , lookup
History of chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical plant wikipedia , lookup
Water splitting wikipedia , lookup
Atomic theory wikipedia , lookup
Rate equation wikipedia , lookup
Chemical potential wikipedia , lookup
Process chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Acid–base reaction wikipedia , lookup
Electrolysis of water wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Click chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Chemical reaction wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Transition state theory wikipedia , lookup
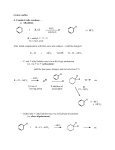
TEST CHEMICAL REACTIONS 2011 Practice Questions 1. The only sure evidence that a chemical reaction has occurred is 2. CaCO3 represents a chemical _____. 3. A shorter, easier way to show chemical reactions, using symbols instead of words, is called a _____. 4. The substances on the left of the arrow in a chemical equation are the substances you start with called ______. 5. Give an example of a change that is NOT a chemical reaction? 6. How many atoms total are in the formula NaHCO3? 7. When chemical reactions occur a. chemical bonds break. c. new bonds form. b. atoms rearrange. d. All of the above 8. The substances formed from a chemical reaction are (the substances you end up with) called ____. Choose the balanced equation for each of the following: 9. a. b. 10. a. b. H2 + O2 H2O H2 + O2 H2O2 c. H2 + O2 2H2O d. Al + HCl AlCl3 + H2 Al + HCl AlCl3 + H2 c. 2Al + 6HCl 2AlCl3 + 3H2 2H2 + O2 2H2O H2 + 2O2 2H2O Al + 3HCl AlCl3 + 3H2 d. Al + 3HCl AlCl3 + H2 11. The law of conservation of matter states that matter cannot be created or destroyed means that a chemical equation must show the same 12. A chemical reaction in which energy is absorbed or taken in is 13. Which reaction requires a continuous supply of energy in order to continue? 14. 15. 16. 17. What is shown by A in Graph 1? What is shown by B in Graph 1? What type of reaction is shown in Graph 1? Which graph illustrates the type of reaction that occurs when wood burns? 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. The minimum amount of energy needed for substances to react is called A covalent bond in which electrons are shared unequally is What happens when an acid reacts with a base? ____ is a compound that increases the number of hydrogen ions (H+) when dissolved in water. Which of the following would taste sour? When all the molecules of a compound break apart in water to make hydrogen ions (H+), you have a ____. When few molecules of a compound break apart in water to make hydrogen ions (H+), you have a _____. ____ is a compound that increases the number of hydroxide ions (OH-) when dissolved in water. A solution that has a pH of ____ is neutral. Some plants, such as pine trees, prefer soil with a pH between 4 and 6. These plants prefer Consider the following reaction: HCl + NaOH H2O + NaCl 28. Identify the acid in the reaction above. 29. Identify the base in the reaction above. 30. Identify the salt in the reaction above.