* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ACTIVATION OF THE COMPLEMENT SYSTEM
Clinical neurochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Gene regulatory network wikipedia , lookup
Proteolysis wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Amino acid synthesis wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Western blot wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Signal transduction wikipedia , lookup
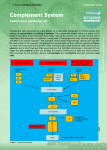
THE COMPLEMENT SYSTEM Complement system The complement system is a set of plasma proteins that act in a cascade to attack and kill extracellular pathogens. Approximately 30 components: - activating molecules - regulator factors - complement receptors - Membran proteins wich inhibit the lysis of host cells Most of the complement proteins and glycoproteins are produced in the liver in an inactive form. Activation is induced by proteolitic cleavage. Enzyme cascades of the plasma: complement and coagulation Markiewski MM 2007 (i) complement activation, (ii) C3-convertase C3a, C3b, (iii) C3a platelet activation, (iv) C3b C5-convertase C5a, C5b, (v) C5a tissue factor, PAI-1 expression, (vi) C5b MAC,…(ix) contact activation (intrinsic pathway) (prekallikrein, factor XII), (x) extrinsic pathway (TF+factor VII), (xi) factor X, (xii) from protrombin (II) to trombin (IIa), (xiii) trombin fibrinpolimerisation (fibrin), (xiv) trombin C3 and C5 breakdown A plazmacascades Enzyme enzim rendszerei of the plasma Coagulation cascade Kallikrein-kinin system Complement cascade Alternative pathway MB-lectin pathway MAC Classical pathway AMPLIFICATION OF THE COMPLEMENT CASCADE limited inactive precursors proteolysis enzyme activating surface Activating sutface needed! ACTIVATION OF THE COMPLEMENT SYSTEM COMPLEMENT SYSTEM CLASSICAL PATHWAY MB-LECTIN PATHWAY ALTERNATIVE PATHWAY COMPLEMENT ACTIVATION RECRUITMENT OF INFLAMMATORY CELLS OPSONIZATION OF PATHOGENS KILLING OF PATHOGENS COMPLEMENT SYSTEM CLASSICAL PATHWAY Antigen-antibody complex C1q, C1r, C1s Serin protease C4, C2 C4a* MB-LECTIN PATHWAY ALTERNATIVE PATHWAY Mannose Pathogen surface MBL MASP-1/MASP-2 C3 B, D Serin protease C4, C2 C3 CONVERTASE C3a, C5a C3b Inflammatory peptid mediators Phagocyte recruitment Opsonization Binding to phagocyte CR Immune complex removal Terminal C5b – C9 MAC Pathogen/cell lysis Classical pathway THE C1 COMPLEX Collagen „legs” Gobular „heads” C1 is always present in serum but it can operate on an activating surface in normal case Low affinity binding to the C-terminal of antibody - Multiple interaction with immune complexes Only in classical pathway! Immunoglobulin Fragments: Structure/Function Relationships antigen binding complement binding site binding to Fc receptors C1 component placental transfer Association between native and adaptive immunity Only the antigen-linked antibodies are able to associate to complement. Why? The classical pathway of complement activation is initiated by binding of C1q to antibody on a bacterial surface GLYCOSYLATION OF PROTEINS IS DIFFERENT IN VARIOUS SPECIES Prokariotic cells Eukariotic cells Mannose Glucoseamine Mannose Galactose Neuraminic acid (sialic acid) MANNAN-BINDING LEKTIN ACTIVATES THE COMPLEMENT SYSTEM THE CENTRAL COMPONENT OF THE COMPLEMENT SYSTEM C3 CGEQ CLEAVAGE SITE One of the proteins present at the highest concentration in serum 1.2mg/ml Is it a lot??? 3 900.000.000.000.000 molecules/ml ACTIVATION OF C3 C3 Active thioester CGEQ CGEQ CGEQ C3a C3b Inflammation Binding OH R OH R O R ROH Cell CGEQ OH OH R RR O Bacterium Membrane attack complex (MAC) • The membrane attack complex affects to the bacterial cell wall, but complement fragments can be attached to the body’s cell surface also • Complement-mediated lysis of the cells is blocked by cell surface and soluble inhibitory factors • Certain bacteria can activate the C3 complement component directly (ALTERNATIVE PATHWAY) • Complement-mediated lysis of bacteria opsonized by antibodies takes place in the absence of alternative pathway also The membrane-attack complex assembles to generate a pore in the lipid bilayer membrane MAC in the cell membrane live and bacteria dead Homologue components of classical and alternative pathways Complement receptors Name Ligand Expression CR1 C3b>C4b, iC3b RBC, Mo/MØ, Gr, B Act-T, FDC C3d, C3dg, iC3b EBV, IFNa, CD23 B, activated T, FDC iC3b> C3dg, C3d ICAM-1, LPS, fibrinogen Mo/MØ, Gr, NK Mo/MØ, Gr, NK CD11c/CD18 iC3b, C3dg, C3d Fibriogen C3aR C3a M, B, Gr, Mo/MØ, Trombocites, simaizom, Neur C5aR C5a,, des-Arg-C5a M, B, Mo/MØ, Trombocites, SMC, Neur C1qR C1q collagen part B, NGr, Mo/MØ, endothel C1qRp C1q Fagocyte CD35 CR2 CD21, CD21L CR3 CD11b/CD18 CR4 The role of complement system in in vivo Lectin and alternative pathway lysis MAC classical pathway C3 C3a C3b C3b C3b C4a C5a C3b C3b opsonization phagocytosis OPSONIZATION C3b bacterium complement receptor macrophage Local inflammatory responses can be induced by the small complement fragments C3a, C4a, and especially C5a Regulation of complement system Factor I a-2macrogl C1Inh DAF C4bp CR1 MCP LECTIN PATHWAY HRF C-pept.ase N CD59 Properdin S-protein DAF positive feedback Fact-H CR1 MCP Factor I membrane protein soluble molecule Major regulating factors of complement system C1Inh: C1-inhibitor (serine-protease inhibitor, it can effect in many steps) Factor H: inhibits C3-konvertase of alternative pathway, co-factor of factor I, cleaves C4b and C3b Properdin: ballasts convertases of alternative pathway DAF: Decay Accelerating Factor MCP: Membrane Cofactor Protein CD59: inhibits the linking of C9 and C8 Regulation of C3 convertase f= FACTOR One of the major function of C1 INHIBITOR C1q binds to IgM on bacterial surface C1q binds to at least two IgG molecules on bacterial surface Binding of C1q to Ig activates C1r, which cleaves and activates the serine protease C1s C1INH dissociates C1r and C1s from the active C1 complex Other functions are on the Figure 33. Regulatory proteins on human cells protect them from complement-mediated attack CD59 prevents assembly of terminal complement components into a membrane pore Problem of xenotransplantation Cascalho M & Platt JL Nat Rev Immunol 2001 • • • The ABO blood group antigens, cell surface carbohydrate components of endothel cells and the CD55/DAFand CD59 molecules are genus specific. Xenotransplantation from minipig – the complement regulators on pig cells can not protect from the attack of the recipient complement system. Transgenic (human CD55) animals – lower cytotoxic activity of human serum xenoreactive antibodies against xenotransplanted cells. (Transplant Proc. 2008 Mar;40(2):551-3 ) Deficiencies of complement system – cascade molecules Deficiencies of complement system – regulatory molecules, receptors Hereditary angioneurotic edema (HANO) (hereditary C1INH defect) • 17-year old boy - severe abdominal pain (frequent sharp spasms, vomiting) • appendectomia – normal appendix • family history of prior illness • immunologist’s suspicion: hereditary angioneurotic edema • level of C1INH: 16% of the normal mean • daily doses of Winstrol (stanozolol) – marked diminution in the frequency and severity of symptoms • purified C1INH intravenously – the infusion relieves the symptoms within 25 minutes Main symptoms: • swellings of skin, guts, respiratory tracts • serious acut abdomenal pain, vomiting • larynx swelling – may cause death Treatment: • IV C1INH • kallikrein and bradykinin receptor antagonists Pathogenesis of hereditary angioneurotic edema activation of XII factor Inhibition by C1INH in many steps • bradykinin and C2-kinin: enhance the permeability of postcapillar venules activation of kallikrein activation of proactivator by contraction of endothel • holes in the venule walls • edema formation cleveage of kininogen to generate bradykinin, vasoactive peptide • C1 is always active without cleveage of C2a to generate C2-kinin, vasoactive peptide cleveage of plasminogen to generate plasmin cleveage of C2 to generate C2a activation of C1 activating surface because plasmine is always active Questions hereditary angioneurotic edema 1. Activation of complement system results in the release of histamine and chemokines, which normally produce pain, heat and itching. Why is the edema fluid in HANE free of cellular components, and why does the swelling not itch? - In HANE, C4b and C2b both generated free in plasma because plasmine always actives the C1 - There are not an activating surface, so C4b are not able to bind to a surface, so it is rapidly inactivated. The concentration of C4b and C2b are relatively low, no C3/C5 convertase is formed. C3 and C5 are not cleaved and C3a and C5a are not generated. After the complement activation histamine do not release which is caused by C3a Without C5a there are not cell recruitment BUT there are C2a-kinin and bradykinin which cause edema. 2. Which complement component levels will be decreased? Why? C2 and C4, because of the continous cleveage by activated C1. Questions hereditary angioneurotic edema 3. Would you expect the alternative pathway components to be low, normal or elevated? C1 plays no part in the alternative pathway. This pathway is not affected. 4. What about the levels of the terminal components? The unregulated activation of the early components does not lead to the formation of the C3/C5 convertase, so the terminal components are not abnormally activated. 5. Despite the complement deficiency in patients with HANE, they are not unduly susceptible to infection. Why not? The alternative pathway of complement activation is intact and these are compensated for by the potent amplification step from the alternative pathway. 6. How might you decide the background of the laryngeal edema (HANO or anaphylactic reaction)? If the laryngeal edema is anaphylactic, it will respond to epinephrine. If it is due to HANO, it will not, C1INH needed. Paroxysmal nocturnal hemoglobinuria (PNH) • acqired clonal mutation of PIG-A gene – no GPI-enchored proteins in the cell membrane • CD59 (upper pic) and CD55 complement regulatory proteins • no CD59 and/or CD55: PNH patients are highly susceptible to complement-mediated lysis (lower pic). • Eleveted levels of TF derived from complement-damaged leukocytes • Treating PNH with a humanized antiC5 antibody GPI= glycosylphosphatidylinositol Paroxysmal nocturnal hemoglobinuria (PNH) symptoms and therapy • haemolytic anaemia (9%) and associated symptoms (35%) • haemoglobin and its products in the urine (26%) • thrombosis (6%): in brain veins, mesentheric veins, vv. hepaticae (Budd-Chiarisyndrome) • transformation to acut lymphoid leukemia (ALL) • aplastic anaemia (13%) • eculizumab (anti-C5 monoclonal antibody) • steroids • Iron replacement • transfusion • bone marrow transplantation