* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Toxoplasmosis
Common cold wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Globalization and disease wikipedia , lookup
Rheumatic fever wikipedia , lookup
Urinary tract infection wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Schistosoma mansoni wikipedia , lookup
Innate immune system wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Infection control wikipedia , lookup
Hepatitis C wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Neonatal infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Schistosomiasis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
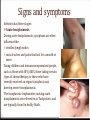
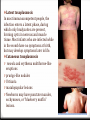
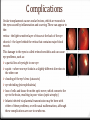
Introduction Epidemiology Pathogenesis Causes Risk factors Signs and Symptoms Prevention Diagnosis Management Complications Case study References Introduction Toxoplasmosis is a parasitic disease caused by the protozoan Toxoplasma gondii. The parasite infects most genera of warm-blooded animals, including humans, but the primary host is the felid (cat) family. Animals are infected by eating infected meat, by ingestion of feces of a cat that has itself recently been infected, and by transmission from mother to fetus. Cats are the primary source of infection to human hosts, although contact with raw meat, especially lamb, is a more significant source of human infections in some countries. Epidemiology T. gondii infections occur throughout the world, although infection rates differ significantly by country. For women of childbearing age, a survey of 99 studies within 44 countries found the areas of highest prevalence are within Latin America (about 50–80%), parts of Eastern and Central Europe (about 20–60%), the Middle East (about 30-50%), parts of Southeast Asia (about 20–60%), and parts of Africa (about 20–55%). Types There are types I, II, and III. These three types of T. gondii have differing effects on certain hosts, mainly mice and humans due to their variation in genotypes. Type I: virulent in mice and humans, seen in AIDS patients. Type II: non-virulent in mice, virulent in humans (mostly Europe and North America), seen in AIDS patients. Type III: non-virulent in mice, virulent mainly in animals but seen to a lesser degree in humans as well. Because the parasite poses a particular threat to fetuses when it is contracted during pregnancy, much of the global epidemiological data regarding T. gondii comes from seropositivity tests in women of childbearing age. Seropositivity tests look for the presence of antibodies against T. gondii in blood. Pathogenesis T. gondii Survival Mechanisms Once the plasmid T. gondii infects a normal host cell, it resists damage caused by the host’s immune system, as well as changing the host's immune processes. One way is by an anti-apoptotic mechanism, allowing the infected host cells to persist and replicate. One method of apoptosis resistance is by disrupting pro-apoptosis effector proteins, such as Bax and Bak. In order to disrupt these proteins, T. gondii causes conformational changes to the proteins. T. gondii does not, however, cause downregulation of the proapoptosis effector proteins. T. gondii also has the ability to initiate autophagy of the host’s cells. This leads to a decrease in healthy, uninfected cells, and consequently less host cells to attack the infected cells. Research by Wang et al finds that infected cells lead to higher levels of autophagosomes in normal and infected cells. Their research reveals that T. gondii causes host cell autophagy using a calcium-dependent pathway. Another study suggests that the parasite can directly affect calcium being released from calcium stores, which are important for the signalling processes of cells. Causes Contact with cat feces that contain the parasite. Cats who hunt or who are fed raw meat are most likely to harbor T. gondii. Eating contaminated food or drink contaminated water. Lamb, pork and venison are especially likely to be infected with T. gondii. Occasionally, unpasteurized dairy products also may contain the parasite. Use of contaminated knives, cutting boards or other utensils. Eating unwashed fruits and vegetables. Receiving an infected organ transplant or transfused blood. In rare cases, toxoplasmosis can be transmitted through an organ transplant or blood transfusion. Risk factors HIV/AIDS. Many people with HIV/AIDS also have toxoplasmosis. In some cases, the infection is recent, and in others, an old infection has become active again. Chemotherapy. Chemotherapy affects your immune system, making it difficult for your body to fight even minor infections. Steroids or other immunosuppressant drugs. Medications used to treat certain nonmalignant conditions suppress your immune system and make you more likely to develop complications of toxoplasmosis. Pregnancy: If you have active toxoplasmosis, treatment can reduce the risk to your baby. If you've already had toxoplasmosis before becoming pregnant, you generally can't pass the infection to your baby. Fecal contamination of hands is a significant risk factor. Signs and symptoms Infection has three stages: Acute toxoplasmosis During acute toxoplasmosis, symptoms are often influenza-like: 0 swollen lymph nodes 0 muscle aches and pains that last for a month or more. Young children and immunocompromised people, such as those with HIV/AIDS, those taking certain types of chemotherapy, or those who have recently received an organ transplant, may develop severe toxoplasmosis. The toxoplasmic trophozoites causing acute toxoplasmosis are referred to as Tachyzoites, and are typically found in bodily fluids. Latent toxoplasmosis In most immunocompetent people, the infection enters a latent phase, during which only bradyzoites are present, forming cysts in nervous and muscle tissue. Most infants who are infected while in the womb have no symptoms at birth, but may develop symptoms later in life. Cutaneous toxoplasmosis 0 roseola and erythema multiforme-like eruptions 0 prurigo-like nodules 0 Urticaria 0 maculopapular lesions 0 Newborns may have punctate macules, ecchymoses, or “blueberry muffin” lesions. Prevention Wear gloves when gardening, particularly when handling soil. Also, be sure to wash your hands thoroughly afterwards with soap and hot water. Do not eat raw, or undercooked, meat, particularly lamb, pork, and venison, including any ready-prepared chilled meals. Avoid sheep and their newborns during the lambing season if you are at extra risk. Wash all kitchenware thoroughly after preparing raw meat. Wash all fruits and vegetables before cooking and eating, including ready-prepared salads. Avoid un-pasteurised goat's milk or products that are made from it. Do not handle or adopt stray cats. Avoid cat faeces in cat litter or soil. Wear gloves if you are changing a cat litter tray, and if you are pregnant, or immune deficient, ask someone else to do this for you. Feed your cat dried, or canned, cat food, rather than raw meat. Don’t!!! Do Diagnosis Diagnosis of toxoplasmosis in humans is made by biological, serological, histological, or molecular methods, or by some combination of the above. Detection of T. gondii in human blood samples may also be achieved by using the polymerase chain reaction. Inactive cysts may exist in a host that would evade detection. Several serological procedures are available for the detection of T. gondii antibody in patients, which may aid diagnosis; these include the SabinFeldman dye test (DT), the indirect hemagglutination assay, the indirect fluorescent antibody assay (IFA), the direct agglutination test, the latex agglutination test (LAT), the enzyme-linked immunosorbent assay (ELISA), and the immunosorbent agglutination assay test (IAAT). The IFA, IAAT and ELISA have been modified to detect immunoglobulin M (IgM) antibodies. IgG antibody The most commonly used tests for the measurement of IgG antibody are the DT, the ELISA, the IFA, and the modified direct agglutination test. These tests reveal that IgG antibodies usually appear within 1–2 weeks of acquisition of the infection, peak within 1–2 months, decline at various rates, and usually persist for life. Acute infections can be differentiated from chronic infections using the “differential” agglutination test (also known as the AC/HS test), which is best used in combination with a panel of other tests such as the TSP. The AC/HS test results when parasites are fixed for use in the agglutination test with two different compounds (i.e., acetone and formalin). The different antigenic preparations vary in their ability to recognize sera obtained during the acute and chronic stages of the infection. IgM antibody The most commonly used tests for the measurement of IgM antibody are doublesandwich or capture igM-ELISA kits, the IFA test, and the immunosorbent agglutination assay (IgM-ISAGA). Commercial test kits often have low specificity, and the reported results are frequently misinterpreted. The IgM antibodies appear sooner after infection than the IgG antibodies and disappear faster than IgG antibodies after recovery. Congenital toxoplasmosis A whole set of recommendations applies for the diagnosis of congenital toxoplasmosis: i) prenatal diagnosis based on molecular testing of amniotic fluid and ultrasound examinations; ii) molecular testing of placenta and cord blood, comparative mother-child serologic tests and a clinical examination at birth; iii) neurologic and ophthalmologic examinations and a serologic survey during the first year of life. Toxoplasmosis cannot be detected with immunostaining. Lymph nodes affected by Toxoplasma have characteristic changes, including poorly demarcated reactivegerminal centers, clusters of monocytoid B cells, and scattered epithelioid histiocytes. Diagnosis of cutaneous toxoplasmosis is based on the tachyzoite form of T. gondii being found in the epidermis. It is found in all levels of the epidermis, is about 6 μm by 2 μm and bow-shaped, with the nucleus being one-third of its size. It can be identified by electron microscopy or by Giemsa staining tissue where the cytoplasm shows blue, the nucleus red. Treatment Acute The medications prescribed for acute toxoplasmosis are the following: Pyrimethamine — an antimalarial medication Sulfadiazine — an antibiotic used in combination with pyrimethamine to treat toxoplasmosis Combination therapy is usually given with folic acid supplements to reduce incidence of thrombocytopaenia. Combination therapy is most useful in the setting of HIV. Clindamycin Spiramycin — an antibiotic used most often for pregnant women to prevent the infection of their children (other antibiotics, such as minocycline, have seen some use as a salvage therapy). Latent In people with latent toxoplasmosis, the cysts are immune to these treatments, as the antibiotics do not reach the bradyzoites in sufficient concentration. The medications prescribed for latent toxoplasmosis are: Atovaquone — an antibiotic that has been used to kill Toxoplasma cysts inside AIDS patients Clindamycin — an antibiotic that, in combination with atovaquone, seemed to optimally kill cysts in mice Trimethoprim/sulfamethoxazole is the drug of choice to prevent toxoplasmosis, but not for treating active disease. Complications Ocular toxoplasmosis causes ocular lesions, which are wounds in the eyes caused by inflammation and scarring. These can appear in the: retina – the light-sensitive layer of tissue at the back of the eye choroid – the layer behind the retina that contains major blood vessels This damage to the eyes is called retinochoroiditis and can cause eye problems, such as: a partial loss of eyesight in one eye squint – where one eye looks in a slightly different direction to the other one clouding of the eye's lens (cataracts) eye shrinking (microphthalmia) loss of cells and tissue from the optic nerve, which connects the eye to the brain, resulting in poor vision (optic atrophy) Infants infected via placental transmission may be born with either of these problems, or with nasal malformations, although these complications are rare in newborns. Antibiotics and steroids are often used to treat the lesions. The scarring caused by toxoplasmosis will not clear up, but treatment may prevent it from getting worse. Congenital toxoplasmosis In most cases, babies born with congenital toxoplasmosis develop normally after treatment with antibiotics. However, in up to 4% of cases, serious complications can develop within the first years of life. These include: permanent visual impairment (partial or, very rarely, complete sight loss) permanent brain damage Death Cases of ocular toxoplasmosis can also occur years after infection. One study found the average age at which it appeared was nine years old. It's also possible for someone to develop complications in their 20s or 30s. These may include: learning disabilities hearing loss ocular toxoplasmosis Encephalitis-toxoplasmosis can lead to seizures and life-threatening illnesses such as encephalitis — a serious brain infection. In people living with AIDS, untreated encephalitis resulting from toxoplasmosis is fatal. Relapse is a constant concern for immunocompromised people with toxoplasmosis. Clinical case A 50-year-old woman presented with sudden onset of decreased vision in her better left eye. She had 1 week of redness, photophobia, pain, and decreased vision. She reported no underlying systemic disease or recent health change and no family history of unusual eye disease. (She was 1 of 8 siblings with the same biological parents.) She was not on medication. She did report an allergy to sulfur medicines that have given her "a bad rash." Of most significance was the fact that she had developed severe vision loss in her right eye about 20 years prior from recurrent attacks "from some type of parasite" in her native country of Brazil. She said that she grew up with a lot of cats that roamed the outdoors and that her family had lived in several regions of Brazil. Her right eye was damaged in a motor vehicle accident at some point after it had developed very poor vision from the parasitic infection. She immigrated to the United States about 10 years ago with her husband. On examination, her visual acuity was light perception in her right eye and 20/100 in her left eye. Her intraocular pressures were 4 mm Hg in her right eye and 14 mm Hg in her left eye. There was no view of the anterior chamber of her right eye secondary to an opaque cornea. However, she had 2+ cell and flare of her left eye. In addition, she had several keratic precipitates in her left eye that were fresh in appearance. There was no view of the posterior pole of her right eye, and an ultrasound was unremarkable, including the lack of any vitreal debris. The vitreous of her left eye had 1+ anterior vitreal cells (with more cells in the posterior vitreous) with some macular edema. Initially, it was difficult to get a complete view of her fundus, but it did appear that there was a focal white area in the superior nasal left fundus adjacent to a darker area. Her chest x-ray was normal. In addition, fluorescent treponemal antibody, complete blood cell count, Creactive protein, erythrocyte sedimentation rate, Lyme, and Bartonella assays were negative. A recent physical exam by her internist, including a purified protein derivative (tuberculin), was negative. However, Toxoplasma gondii immunoglobulin (Ig)G returned with a high positive titer. References www.mayoclinic.org www.hse.ie http://www.nhs.uk/ http://en.wikipedia.org/ https://www.google.com http://www.medscape.org/ http://www.kellogg.umich.edu/