* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topic 14
Canonical quantization wikipedia , lookup
Renormalization group wikipedia , lookup
EPR paradox wikipedia , lookup
Tight binding wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Renormalization wikipedia , lookup
Hidden variable theory wikipedia , lookup
Dirac equation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Path integral formulation wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Ensemble interpretation wikipedia , lookup
Electron scattering wikipedia , lookup
Atomic theory wikipedia , lookup
Schrödinger equation wikipedia , lookup
Wheeler's delayed choice experiment wikipedia , lookup
Identical particles wikipedia , lookup
Elementary particle wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Probability amplitude wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Particle in a box wikipedia , lookup
Wave function wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
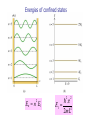
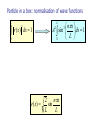
PHY 102: Waves & Quanta Topic 14 Introduction to Quantum Theory John Cockburn (j.cockburn@... Room E15) •Wave functions •Significance of wave function •Normalisation •The time-independent Schrodinger Equation. •Solutions of the T.I.S.E The de Broglie Hypothesis In 1924, de Broglie suggested that if waves of wavelength λ were associated with particles of momentum p=h/λ, then it should also work the other way round……. A particle of mass m, moving with velocity v has momentum p given by: p mv h Kinetic Energy of particle 2 2 2 2 p h k KE 2 2m 2m 2m If the de Broglie hypothesis is correct, then a stream of classical particles should show evidence of wave-like characteristics…………………………………………… Standing de Broglie waves Eg electron in a “box” (infinite potential well) V= V= V=0 Electron “rattles” to and fro V= V= V=0 Standing wave formed Wavelengths of confined states In general, k =nπ/L, n= number of antinodes in standing wave 2L 3 ;k 3 L 2 L;k L 2L ; k L Energies of confined states k n E 2 2m 2mL 2 2 2 En n 2 E1 E1 2 2mL 2 2 2 2 Energies of confined states En n E1 2 E1 2 2mL 2 2 Particle in a box: wave functions From Lecture 4, standing wave on a string has form: y ( x, t ) ( A sin kx) sin( t ) Our particle in a box wave functions represent STATIONARY (time independent) states, so we write: ( x) A sin kx A is a constant, to be determined…………… Interpretation of the wave function The wave function of a particle is related to the probability density for finding the particle in a given region of space: Probability of finding particle between x and x + dx: ( x ) dx 2 Probability of finding particle somewhere = 1, so we have the NORMALISATION CONDITION for the wave function: ( x) 2 dx 1 Interpretation of the wave function Interpretation of the wave function Normalisation condition allows unknown constants in the wave function to be determined. For our particle in a box we have WF: nx ( x) A sin kx A sin L Since, in this case the particle is confined by INFINITE potential barriers, we know particle must be located between x=0 and x=L →Normalisation condition reduces to : L ( x) 0 2 dx 1 Particle in a box: normalisation of wave functions ( x) 0 nx A sin dx 1 L 0 L L 2 dx 1 2 2 nx ( x) sin L L 2 Some points to note………….. So far we have only treated a very simple one-dimensional case of a particle in a completely confining potential. In general, we should be able to determine wave functions for a particle in all three dimensions and for potential energies of any value Requires the development of a more sophisticated “QUANTUM MECHANICS” based on the SCHRÖDINGER EQUATION………………… The Schrödinger Equation in 1-dimension (time-independent) d ( x) V ( x ) ( x ) E ( x ) 2 2m dx 2 2 KE Term PE Term Solving the Schrodinger equation allows us to calculate particle wave functions for a wide range of situations (See Y2 QM course)……. Finite potential well WF “leakage”, particle has finite probability of being found in barrier: CLASSICALLY FORBIDDEN Solving the Schrodinger equation allows us to calculate particle wave functions for a wide range of situations (See Y2 QM course)……. Barrier Penetration (Tunnelling) Quantum mechanics allows particles to travel through “brick walls”!!!! Solving the SE for particle in an infinite potential well V ( x) 0 0xL So, for 0<x<L, the time independent SE reduces to: 2 d 2 ( x) E ( x) 2 2m dx d 2 ( x) 2mE ( x) 0 2 2 dx General Solution: 1/ 2 2mE ( x) A sin 2 1/ 2 2mE x B cos 2 x 1/ 2 2mE ( x) A sin 2 1/ 2 2mE x B cos 2 x Boundary condition: ψ(x) = 0 when x=0:→B=0 1/ 2 2mE ( x) A sin 2 x Boundary condition: ψ(x) = 0 when x=L: 1/ 2 2mE (0) A sin 2 n E 2 2mL 2 L0 2 2 nx ( x) A sin L In agreement with the “fitting waves in boxes” treatment earlier………………..