* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atoms and Elements Notes
Oxidation state wikipedia , lookup
Bent's rule wikipedia , lookup
Livermorium wikipedia , lookup
Photoelectric effect wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electrochemistry wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Condensed matter physics wikipedia , lookup
Chemical element wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Periodic table wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Atomic orbital wikipedia , lookup
History of chemistry wikipedia , lookup
Electronegativity wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Bond valence method wikipedia , lookup
Atomic nucleus wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
History of molecular theory wikipedia , lookup
Extended periodic table wikipedia , lookup
Electron configuration wikipedia , lookup
Metallic bonding wikipedia , lookup
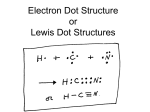
Atoms • Atom- The smallest particle that can be called an element. • All matter is made up of atoms. • Made up of Protons(+), Neutrons, and Electrons (-) Elements • A pure substance • All atoms of an element have the same # of protons. • An element can not be broken into another substance • No two elements are the same in their properties Element Examples 1. 2. 3. 4. Element Hydrogen Oxygen Nitrogen Carbon Made up of Elements 1. Water 2. Air 3. Rock Concentrations The most abundant element in: The Universe Hydrogen (H) – 98% In the Earths Crust Oxygen- 47% Silicon- 28% Three Atomic Particles 1. Proton a. Found in the Nucleus b. Positively Charged (+) c. Is the Atomic #/whole # which = identity (C=6) 2. Electron a. Found in a cloud around the Nucleus b. Negative Charge (-) c. Same # as the Atomic Number (C = 6) d. Can be lost or gained to create charged particles (ions). e. Determine how elements will bond/react. 3. Neutrons a. Found in the Nucleus with the Protons. b. Neutral/No charge c. Creates Isotopes which are different versions of the same element (i.e.C14 vs. C12) d. Can be calculated by subtracting the atomic # from the atomic mass. Decimal # rounded - Whole # = # of neutrons (i.e. C = 12 – 6 = 6 neutrons) Properties of Matter 1. Physical Property- How a substance looks, feels, tastes, etc. Ex. Sugar is white, sweet and granular. 2. Chemical Property- How a substance reacts to the world round it. Ex. Sugar will turn to a black solid when mixed with Sulfuric Acid. How Matter Changes Conservation of Mass- Matter is neither created nor destroyed it just changes form. 1. Physical Change- A change in form that does not result in a new substance. Ex. Sugar dissolves in water or any state change. 2. Chemical Change- A change/reaction that creates a new substance. An indication this is happening is when you see a color change, heat, light, smoke/gas and a new byproduct. Ex. Oxidation (rust), Combustion (burning) or any Photosynthesis and Cellular Respiration. Metals Physical 1. Shininess 2. Conduct Heat and Electricity 3. High Density (Heavy) 4. Ductile (Can be made into wire) 5. Malleable (Can be flattened) 6. All solids except Hg and H Chemical 1. Tend to lose electrons to make positive ions. 2. Tend to bond with non-metals like oxygen and water.(Corrosion) Non Metals Physical 1. Not Shiny 2. Do not conduct electricity or heat Chemical 1. Tend to gain 3. Low Density electrons to (lighter) make negative 4. Brittle (not ductile ions or malleable) 5. Can be gas, solid 2. Tend to bond with metals or liquid Alkali Metals 1. First Column 2. Never found alone in nature because they are so reactive. 3. React violently with water due to only having 1 valence electron Halogens 1. Second to last Column 2. Never found alone in nature 3. Highly reactive with Alkali Metals due to 7 electrons in the valence shell Nobel Gases 1. Last Column 2. Do not react with anything due to having 8 valence electrons 3. All gases Valence Electrons • Electrons in the outer most shell • Can be determined by the column # (group) that the element exists in. • All atoms trying to reach the magic 8 number of valence electrons. (Octet Rule) • Number of valence electrons determines how reactive an element is and what type of bonds they form. Ionic Bonding • An ion is formed when an atom gives up an electron and becomes positive (+) or an atom gains an electron an becomes negative (–) • An ionic bond is formed when two opposite charged atoms come in contact and stick to together. • This the strongest of the chemical bonds Covalent Bonding • Bond created when two atoms share electrons • Not as strong as ionic bonds (easier formed and easier broken) • Carbon makes a lot of these Electron Dot Model 1. Determine the # of valence electrons 2. Draw the atomic symbol 3. Starting at “12 o’clock” place a dot at 12, 3, 6 and 9 4. If there are more than 4 valence electrons you then start pairing electrons at each spot. 5. Bonding sites are all non-paired electrons