* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download microscopy technique-2
Extracellular matrix wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
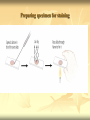
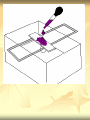
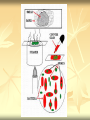
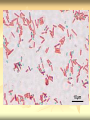
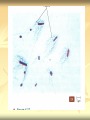
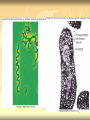
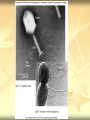
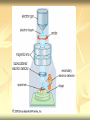
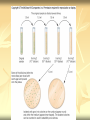
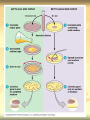
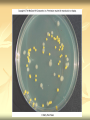
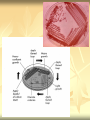
ERT107 MICROBIOLOGY FOR BIOPROCESS ENGINEERING Pn Syazni Zainul Kamal PPK Bioprocess Chapter 2: Microscopy Techniques CO2 : Ability to demonstrate practices in microscopy, staining, sterilization, isolation and identification of bacteria and fungi Purpose of STAINING? STAINING Microorganisms must be fixed and stained prior examined under microscope to : - increase visibility (increase contrast) - accentuate specific morphological features - preserve them for future study Staining – coloring specimens with stain (dyes) Preparing specimens for staining Prior to staining, specimens need to be smear and fixed which involve following steps : making a thin film (smear) of the specimen on a slide The smear is air dried Fixation Microorganism is killed & attached firmly to microscope slide (heat fixation) passing the slide through a flame (preserve overall morphology but not structure within cell, bacteria, archae) OR (chemical fixation) applying a chemicals (ethanol, acetic acid, formaldehyde) to attach the specimen to the slide (used to protect overall morphology & subcellular structure, larger microbes) Preparing specimen for staining Dyes make internal and external structures of cell more visible by increasing contrast with background have two common features : 1) chromophore groups chemical groups with conjugated double bonds give dye its color 2) ability to bind cells Commonly used dyes (ionizable dyes): 1) Basic dyes - methylene blue, basic fuchsin, crystal violet - have +ve charged groups, bind to –ve charged eg nucleic acids, proteins, surface of bacteria 2) acidic dyes - eosin, rose bengal, acid fuchsin - have –ve charged groups, bind to +ve charged cell structure Simple staining for microorganisms 1) 2) 3) 4) Prepare bacterial fixed smear on slide Place a slide on staining tray and flood the smear with dyes using appropriate exposure time Gently wash the excess stain off with water Blots the slide dry (do not wipe) Simple staining to determine the size, shape, arrangement of procaryotic cells Differential staining Differential staining – a series of more than one dye distinguish organisms based on their staining properties (gram staining, acid-fast staining) Detect presence/absence of structures (flagella, capsule, endospore) (1) Gram staining - developed in 1884 by Christian Gram. - widely used today - divides bacteria into 2 classes ; gram positive & gram negative - result – gram positive (purple), gram negative (pink) Procedures for GRAM STAINING (2) Acid-fast staining Commonly used to stain cells of the genera Mycobacterium tuberculosis and Nocardia (have cell wall with high lipid content) Developed by Franz Ziehl and Friedrish Neelsen Ziehl-Neelsen acid fast staining Acid-fast staining procedures : 1) Flood the slide with the red primary stain, carbolfuchsin, for several minutes while warming it over steaming water. Heat is used to drive the stain through the waxy wall & the cell, where it remains trapped. 2. Cool the slide 3. Decolorize the smear by rinsing it with a solution of acid-alcohol. *Result from acid-alcohol bleaching action: - remove color from non acid-fast cells - Acid-fast cells retain their red color because the acid cannot penetrate the waxy wall. 4. Counterstain with methylene blue, which stains only bleached, non-acid-fast cells. 5. Rinse slide with water to remove excess methylene blue 6. Blot dry 3. Capsule staining Reveals the presence of capsules (made of polysaccharides), B.anthracis Dyes used : india ink, eosin, nigrosin (acidic dyes) Mix dye with cell & make a smear over the slide Air dried Cell appear brighter again dark background Acidic dyes stain the background & does not penetrate the capsule The bacteria cell had been counterstained with basic dyes 4. Endospore staining Endospore - dormant, tough and temporarily nonreproductive structure produced inside the cytoplasm by certain bacteria eg. Bacteria from genera Bacillus and Clostridium. Can survive over heat, extreme chemical and desiccation Spherical/elliptical/either smaller or larger than the diameter of parent bacterium. Not stained well by most dyes. Need a harsh treatment to drive the dye into endospore Schaeffer-Fulton 1) Smear the organism and heat fix to a slide 2) Place the slide over a steam bath and flood with Malachite Green 3) Keep the stain over the bath for 3 - 5 minutes, recovering the slide with Malachite Green if some evaporates. 4) Dump the Malachite Green off and allow to cool 5) Rinse the slide with water to remove excess stain 6) Counterstain the smear with Safranin for 2 minutes 7) Rinse the slide with water to remove excess stain 8) Blot dry the stain and view under a microscope Step Finished Color of Vegetative Cell Color of Endospore Smear Colorless Colorless Malachite Green Green Green Cool/Wash Colorless Green Safranin Safranin Green 5. Flagella staining Provide information on the presence and distribution pattern of flagella on procaryote cells Procaryote flagella – fine, threadlike organelles of locomotion that are so slender (10-30mm). Can only be seen directly using EM So, to observe using light microscope -increase thickness of the flagella Procedures : 1) coated with mordants eg. Tannic acid & potassium alum (increase diameter) 2) stain with pararosaniline or basic fuschin (change colour & increase contrast) Electron Microscopy Electron microscopy Light microscope resolution limit of about 0.2µm. Limits to detailed studies of many microorganisms. Eg. Viruses & internal structure of microorganisms shortest wavelength of visible light is about 400 nm Electrons have wavelengths between 0.01 nm and 0.001 nm; thus, their resolving power is much greater, and they typically magnify objects 10,000× to 100,000×. EM – use beam of electron to illuminate and create magnified images of specimen There are two general types of electron microscope: Transmission Electron Microscopes (TEM) Scanning Electron Microscopes (SEM) TEM A TEM generates a beam of electrons that passes through a thinly sliced & dehydrated specimen, through magnetic fields that manipulate and focus the beam, and then onto a fluorescent screen that changes the electron’s energy into visible light. Column of the TEM must be vacuum - electrons are deflected by collisions with air molecules TEM TEM specimen must be very thin (20 to 100nm) (solid matter easily absorb and deflect electron) - fixation of specimen using glutaraldehyde - specimen need to be dehydrated - embedded in plastic - slice thinly Preparing sample for TEM 1) fixation of specimen using chemicals eg glutaraldehyde or osmium tetraoxide (stabilize cell structure) Dehydrated with organic solvent eg. Acetone or ethanol Specimen soaked in unpolymerized, liquid epoxy plastic, hardened and form solid block. Cut to a thickness of 100nm with diamond knife mounted in a slicing machine (ultramicrotome) Soak thin section with heavy metal eg lead citrate or uranyl acetate (bind to cell & make it more electron opaque, increase contrast) Place thin section on copper grid n ready to view 2) 3) 4) 5) 6) Other Preparation Methods negative stain heavy metals (phosphotungstic acid/ uranyl acetate) do not penetrate the specimen but render dark background used for study of viruses, bacterial gas vacuoles shadowing coating specimen with a thin film of a heavy metal (platinum) only on one side useful for viral morphology, flagella, DNA freeze-etching freeze specimen using liquid nitrogen then fracture along lines of greatest weakness (e.g., membranes) Shadowed & coated using platinum & carbon allows for 3-D observation of shapes of intracellular structures SEM uses electrons reflected from the surface of a specimen to create detailed image produces a realistic 3-dimensional image of specimen’s surface features SEM use magnetic lenses to focus a beam of primary electrons Primary electrons are scanned across the metal-coated surface of a specimen – produced secondary electrons Secondary electrons are collected by a detector & their signal amplified and displayed on a monitor 1) 2) 3) 4) Preparing sample for SEM Fixed specimen using chemicals Dehydrated using aseton & ethanol Dried sample in critical point drying for 24hrs (to preserve surface structure and prevent collapse of the cells) Mounted and coated specimen with a thin layer of metal eg platinum or gold SEM or TEM? ISOLATION Isolation of microorganisms Natural habitat, microorganism grow in complex, mixed populations with many spp. Need a pure culture to study and characterize an individual species Pure culture =contain one type of microorganisms Techniques to prepare pure culture: -spread plate -streak plate -Pour plate Spread plate Small volume of diluted microbial mixture (30-300 cells) transferred to the center of agar plate. Spread evenly over the surface with sterile bent-glass rod. The dispersed cells develop into isolated colonies Pour plate Original sample is diluted several times – reduce microbial population Small vol. of diluted sample mixed with melted agar After agar had hardened, each cell is fixed in place and form individual colony Streak plate An inoculum is spread across the surface of an agar plate in a sequential pattern of streak (as indicated by the numbers n arrows) The loop is sterilized between streaks In streak 2,3 and 4, bacteria are picked up from previous streak, diluting the number of cells each time These develop into separate colonies Microbial Growth on Solid Surfaces colony characteristics that develop when microorganisms are grown on agar surfaces aid in identification Colony – a macroscopically visible cluster of microorganisms differences in growth rate from edges to center is due to oxygen, nutrients, and toxic products cells may be dead in some areas 1. Form – The form refers to the shape of the colony. 2. Elevation – This describes the “side view” of a colony. 3. Margin – The margin or edge of a colony (or any growth) may be an important characterisic in identifying an organisms.