* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Developmental Toxicology
Nicotinic acid adenine dinucleotide phosphate wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Oncogenomics wikipedia , lookup
Epigenomics wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Microevolution wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
History of genetic engineering wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Point mutation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Designer baby wikipedia , lookup
Birth defect wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Genomic imprinting wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
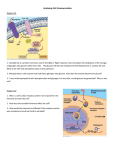
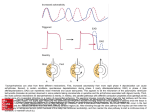
Developmental Toxicology 4 manifestations of developmental toxicity •Structural malformations •Growth retardation •Functional impairment •Death of the organism Teratology 1. the study of malformations or serious deviations from the normal type in organisms 2. the branch of science concerned with the production, development, anatomy, and classification of malformed fetuses. Teratogen Any agent that causes a birth defect After Greek “monster creating” •Environmental conditions (1200) •Maternal nutritional deficiencies (1930) •Rubella virus infection (1941) •Thalidomide (1961) Adverse Outcomes in Pregnancy 1. 57% of pregnancies detected by hCG at 8-9 days after fertilization do not develop as clinically detectable pregnancies 2. 15-20% of recognisable pregnancies end in spontaneous abortion – 90% in the first trimester 3. 2% of pregnancies end in miscarriage >20 weeks. 4. 2-3% of newborn have a major malformation severe enough to require hospitalisation. Cause of human birth defects • • • • • 15-25% genetic cause 4% maternal conditions 3% maternal infections 1-2 deformation (mechanical problem) <1% chemical and environmental influences • 65% unknown etiology Chemical teratogenicity • • • • • • >4100 chemicals have been tested 66% non-teragenic 7% teratogenic more than one species 18% teratogenicin most species tested 9% equivocal experimental results 50-60 chemicals or conditions alter prenatal development in humans (Table 10-1) Pregnancy Risk Categories From: A Textbook of Psychopharmacology. Ed. by Schatzberg and Nemeroff. American Psychiatric Publishing Company, 2004. Therapeutic Drugs Teratogenic to Humans • Anticonvulsants – Phenytoin, primidone, trimethadione, valproic acid, carbamazepine • Anticancer agents – Alkylating agents –busulfan, cyclophosphamide, chlorambucil, mechlorethamine – Antimetabolites-aminopterin, methotrexate, cytarabine • • • • Androgenic hormones-danazol Coumarin anticoagulants-warfarin Retinoids-accutane, isotretinoin, etretinate, acitretin Antihyperlipidemic agents-lovastatin, atorvastatin • Other drugs-diethystilbestrol, thalidomide, penicillamine, lithium, fluconazole, misoprostol Thalidomide Thalidomide • • • • Susceptible period- 20-36 days after fertilization Proposed mechanisms (>24) Embryonal DNA oxidation (PBN can prevent) Misregulation of the expression of genes critical for outgrowth of limb – The inability of NF-kappaB, a redox-sensitive transcription factor, to bind to its DNA promoter results in the failure of limb cells to express fibroblast growth factor (FGF)-10 and twist in the limb progress zone mesenchyme, which in turn attenuates expression of FGF-8 in the apical ectodermal ridge. Diethylstilbesterol (DES) • DES was prescribed between 1940 and 1970 to prevent miscarriages in high risk pregnancies. • DES increases estrogen and progesterone synthesis by the placenta. • In the mid 1970 cases of vaginal adenocarcinoma in women ages 16-20 were linked to fetal exposure through maternal DES ingestion early in the pregnancy. • Female children - vaginal and cervical carcinomas, uterine anomalies. • Male offspring - epididymal cyst, hypotrophic testes, decreased semen volume and poor semen quality. Alcohol (Ethanol) Fetal Alchohol Syndrome (FAS) Fetal Alchohol Effects (FAE) •Cranial facial dysmorphism •Intrauterine and postnatal growth retadation •Retarded psychomotor and intellectual development •IQ 68 Tobacco smoke • • • • Spontaneous abortions Perinatal deaths Lower birth weight Increased risk of • • • • • Sudden infant death syndrome Behavioral attention disorders Orofacial cleft (particular xenobiotic gene polymorphisms) Gastroschisis (with variant alleles N053, ICAM1, NPPA) Branching morphogenesis and maturation of the lung • Nicotine-related adverse nerodevelopmental outcomes Cocaine •At risk for premature labor, spontaneous abortion, increased perinatal mortality and fetal death. •intrauterine growth retardation, microcephaly, altered presencephalic development, decreased birth weight, a neonatal neurologic syndrome of abnormal sleep, tremor, poor feeding, irritability, and occasional seizures. •Genitaouinary tract malformation •Impaired uditory process Retinoic Acid c acid Retinoic acid is the active ingredient in “Accutane”, a drug used to treat severe acne. Since its introduction in September of 1982, an estimated 160,000 women of child bearing age have ingested the drug. Between 1982 and 1987, approximately 900-1300 malformed children, 7001000 spontaneous abortions and 5000-7000 elective abortions are due to Accutane exposure. Exposed children may have hydrocephaly, ear malformations, cardiovascular defects and decreased IQ. Accutane carries a pregnancy category X warning, meaning it is a known human teratogen. Retinoids • Malformations of the face, limbs, heart, CNS, and skeleton • RXR α receptor • Schizophrenia Retinoid Therapies Use Drugs Psoriasis Tazartene (Zorac), Etritinate (Tegison) Adapalene (Differin), Tretinoin (Renova), Isotretinoin (Accutane) Tretinoin/ATRA (Vesanoid) Acne Leukemia RAR and RXR (Simple Version) • Nuclear Receptors (like ER, PPAR, VDR and others) • RXR/RAR Heterodimer is functional unit • Bind selectively to REs in genome • Act as transcription factors • Up-regulate or Repress the expression of particular genes Valproic acid was released in 1967 in Europe and in 1978 in the United States to treat epilepsy. Approximately 11,500 epileptic women become pregnant each year, many of which use valproic acid. By 1980, publications began linking malformed children to in utero exposure to valproic acid (greater than 500 mg/day). Valproic Acid • spina bifida with menigomyelocele or menigocele • The proposed mechanism of action is that valproic acid influences folate metabolism Angiotensin Converting enzyme inhibitors and angiotensin antagonists • 2-3 trimester • related reduced amniotic fluid volume and impaired fetal renal function – – – – – – Oligohydromnios Fetal growth retardation Pulmonary hypoplasia Renal failure Hypotension Death • First trimester – Congenital malformation Wilson’s General Principles of Teratology (Table 10-2) 1. Susceptibility to teratogenesis depends on the genotype of the conceptus and the manner in which this interacts with environmental factors. 2. Susceptibility to teratogenic agents varies with the developmental stage at the time of exposure. 3. Teratogenic agents act in specific ways (mechanisms) on developing cells and tissues to initiate abnormal embryogenesis (pathogenesis). 4. The final manifestations of abnormal development are death, malformation, growth retardation, and functional disorder. 5. The access of adverse environmental influences to developing tissue depends on the nature of the influences (agent). 6. Manifestations of deviant development increase in degree as dosage increases from the no-effect to the totally lethal level. Critical periods of susceptibility and endpoints of toxicity 1. Gametogenesis and Fertilization Mechanism unclear, may be related to imprinting Cytosine methylation and change in chromatin conformation 受精後6hr暴露ethylene oxide, ethylmethane sulfonate, ethylnitrosourea→malformed fetus Early development: ovulation to implantation – DNA Methylation • Methyl groups may be attached to cytosine (C5 position) – Methyltransferases • Methyl groups provide a tag concentrated in CG-rich domains, often in promoter regions Maintains a gene in inactive state rather than initiating gene repression – Example: •Inactivation of genes of one X chromosome in female mammals occurs prior to a wave of methylation •Implantation – a new wave of methylation occurs •Early Zygote – most methylation tags removed DNA Methylation vs Genomic Imprinting • Certain genes are active or inactive during early development – Depending on whether they are paternal or maternal genes – Eg – IGF-2 is only active in the gene from the male parent – The gene is imprinted according to parental origin • Mammalian genome has > 100 imprinted genes in clusters • The majority of imprinted genes in mammals have been found to have roles in the control of embryonic growth and development, including development of the placenta • Imprinted due to selective methylation of one of the alleles 2.Preimplantation著床前期 (blastocyst) 囊胚形成,細胞分裂到1000個細胞,僅3個細胞將 發育成胎兒,餘發育成胎盤等支持組織,在此期暴 露,理論上不影響或稍微影響胎兒生長,不然就導 致死胎。 DDT, nicotine, methylmethane→body and/or brain weight deficits and embryo lethality but not malformation 然而, Methylnitrosourea, cyproterone→malformation Blastocyst The developing embryo becomes a hollow ball of cells and is called a blastocyst. Group of cells within the hollow space forms the inner cell mass (ICM). develops into the embryo. The cells around the ICM become the extraembryonic membranes role in implantation supports embryo’s growth 3. Implantation 著床 第6-13days 4. Gastrulation-三胚層形成, 第3週 在此期暴露有害 物質將造成眼、 腦及臉部的畸形 5. Organogenesis 器官形成,第3-8週 為最容易受影響的時期,因為本期 •Cell proliferation •Cell migration •Cell-cell interactions •Morphogenetic tissue remodeling 6. Fetal period胎兒期 第8wk-birth 在此期暴露,影響生長和功能的成熟,需要在 出生後仔細觀察才能察覺。如中樞神經的異常 包括行為、智力、運動的缺失,生殖力降低, 以及免疫系統、心臟、肺臟、腎臟功能受損等。 *若有構造的改變乃是破壞原本正常的構造稱為 deformation,不同於前述malformation Critical periods of sensitivity for induction of various defect by retinoic acid in the hamster Dose-response Patterns and the threshold concept Mechanisms and pathologenesis of developmental toxicology • • • • • • • • • Mutations Chromosomal breaks Altered mitosis Altered nucleic acid integrity or function Diminished supplies or precursors of substrates Decreased energy supplies Altered membrane characteristics Osmolar imbalance Enzyme inhibition Example of cyclophosphamide (CP) A teratogenic chemotherape utic agent Damage to DNA inhibit cell cycle progression cell cycle arrest too long apoptosis Bind to protein Single strand DNA break CP induces DNA damage (predominant occur in S phase) leading to Cell cycle perturbation Cell death Sensitivity is determined by cell cycle length and cell predisposition to apoptosis Cell death in the neural tube by CP Sensitivity to CP-induced cell death Neuroepithelium >heart Cell cycle length 9.5 hr vs 13.4 hr (longer Go/G1) Advances in the Molecular basis of dysmorphogenesis 1.Using either singly or double gene knockout Retinoic acid receptor family (syndactyly) 2. Antisense oligonucleotide Wnt-1, Wnt-3a (mid and hindbrain malformation) 3. Reporter transgenes RA activate hoxb-1-lacZ Pharmacokinetics and metabolism in pregnancy 1.Changes in maternal physiology hepatic metabolism, GI tract, cardiovascular system, excretory system, respiratory system 2.Overall decrease in hepatic xenobiotic transformation 3.Roles of placenta in influence embryonic exposure help to regulate blood flow -offer a transport barrier-pH gradient, weak acid rapidly transfer -metabolize chemicals 2-acetylaminofluorene (proteratogen) 7-hydroxyl metabolites(proximate teratogen) 4.Maternal metabolism of xenobiotics 2-methoxyethanol 2-methoxyacetic acid Maternal factors affecting development •Genetics high incidence of cleft lip/palate in white mother •Disease-chronic hypertension diabetes infection-cytomegalovirus, Taoxoplasma gondii Hyperthermia-CNS malformation •Nutrition-folate neural tube defect •Stress-noise, restraint •Placenta toxicity -46 toxicants, Cd Placental toxicity • Metals, Cd, As, Hg, ethanol, cocaine, cigaratte, sodium salicylate • Maternal injection vs fetal injection of Cd • Production of metallothionein • Interaction with Zn Maternal toxicity• acetazolamide inhibits carbonic anhydrase forelimb ectrodactyly • diflunsial results in anemia skeleton defects in rabbits • phenytoin affects folate metabolism and heart rates • metallothionein synthesis inducer-urathane, mercaptopurine, valproic acid Zn deficiency Develpmental toxicity of endocrine-disrupting chemicals Definition of endocrine-disrupting chemicals “Exogenous agent that interferes with the production, release, transport, metabolism, binding, action, or elimination of natural hormones responsible for the maintenance of homeostasis and the regulation of developmental processes.” Endocrine-disrupting chemicals Four modes of action 1. Serving as steroid receptors ligands 2. Modifying steroid hormone metabolizing enzymes 3. Perturbing hypothalamic-pituitary release of trophic hormones 4. Uncharacterized proximate modes of action Fetal Basis and Transgenerational Transmission of Reduced Fertility Endocrine Disruptor F0 F1 F2 F3 F4 Vinclozolin (antiandrogenic fungicide) Methoxychlor (estrogenic pesticide) F0=gestating mother F1=1st generation Environmental Epigenetics Anway. Science 2005; 308:1466 Decreased spermatogenic capacity and decreased fertility ..as well as increased prevalence of other diseases transferred via MALE germ line Summary • A transient embryonic exposure to endocrine disruptors at the time of gonadal sex determination can cause epigenetic transgenerational disease state of subfertility and spermatogenic defects in F1 through F4 generations • Transgenerational disease phenotype was primarily transmitted through the male germ line • Exposure appears to have caused an epigenetic reprogramming of the germ cell line that is “permanent” and transferred transgenerationally to subsequent generations Modern safety assessment • Regulatory guidelines for in vivo testing • Multigeneration tests • Children’s health and the food quality protection act – Tenfold safety factor for children • • • • Alternative testing strategies Epidemiology Concordance of data (among species) Elements of risk assessment use-in pregnancy rating: A, B, C, D, X In Vivo Regulatory Protocol Guideline The 17 intercellular signaling pathways by most metazoans • • • • • • • • • • • • • • • • • • • • • Early development and later 1. Wnt pathway 2. Receptor serine/threonine kinase (TGFb) pathway 3. Hedgehog pathway 4. Receptor tyrosine kinase (small G proteins) pathway 5. Notch/Delta pathway Mid-development and later 6. Cytokine receptor (cytoplasmic tyrosine kinases) pathway 7. IL1/Toll NFkB pathway 8. Nuclear hormone receptor pathway 9. Apoptosis pathway 10. Receptor phosphotyrosine phosphatase pathway Larval/adult physiology 11. Receptor guanylate cyclase pathway 12. Nitric oxide receptor pathway 13. G-protein coupled receptor (large G proteins) pathway 14. Integrin pathway 15. Cadherin pathway 16. Gap junction pathway 17. Ligand-gated cation channel pathway Sonic Hedge-hog signal pathway Cholesterol synthesis inhibitor cyclopamine jervine Holoprosencephaly The signalling molecule Sonic hedgehog Shh. Homologue of Drosophila hedgehog gene (involved in compartmentalisation of wing). Three vertebrate homologues, Sonic hedgehog, Indian hedgehog and Desert hedgehog. Secreted molecules, recognised by transmembrane receptors (e.g. Patched1,2). Sonic hedgehog expressed first in notochord, then floor plate. Shh misexpression in dorsal neural tube leads to ectopic floor plate and motor neurons. Inhibition of Shh, or gene knockout, leads to absence of floor plate and motor neurons. Cells ‘read’ the Shh concentration to which they are exposed. Hi [Shh] induces floor plate. 5x lower [Shh] induces motor neurons. Consequences of Folate Deficiency • Result of low dietary intake, genetic error of folate metabolism, lifestyle exposures 1. DNA Hypomethylation − Gene overexpression, uncontrolled cell growth, genomic instability 2. Hyperhomocysteinemia − Excessive accumulation of Hcy 3. Base Misincorporation − Decrease in thymine synthesis; replaced by uracil − DNA strands prone to nicks, breaks and vulnerable to mutagen insertion Homework 1. Describe the possible mechanisms for teratogenic effects of the following chemicals. a. aminoglycosides b. ethylene oxide c. captopril d. danazol e. aminopterin f. Accutane