* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell Signalling
Electrophysiology wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Synaptogenesis wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
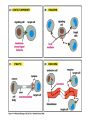
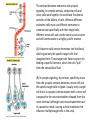
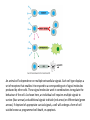
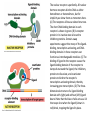
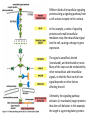
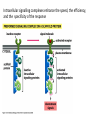
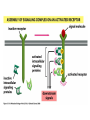
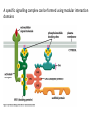
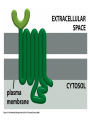
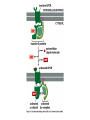
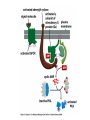
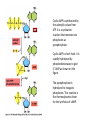
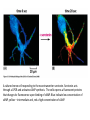
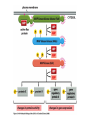
Cell Signalling M. Metodiev 2011/2012 To make multicellular organisms cell must communicate. This communication is mediated by extracellular signal molecules. Sofisticated mechanisms control which signal molecules are released from a specific type of cell, at what time and concentration they are secreted, and how these signals are interpreted by the target cells Some signalling molecules act over long distances, some act only on the immediate neighbour cells Most cells in higher organisms are both emiters and receivers of signals Lecture 1: General Principles of Cell Signalling • Signals, receptors and mediators • The prototypical pheromone signalling pathway of budding yeast • Cell surface and intracellular receptors • Types of intercellular signalling • Nuclear receptor signalling • Types of cell surface receptors • Molecular switches: signalling through GTPases and protein phosphorylation Lecture 2: Discovery and elucidation of novel signalling pathways: a case study Budding yeast cells responding to mating factor. (A) The cells are normally spherical. (B) In response to mating factor secreted by neighbouring yeast cells, they put out a protrusion toward the source of the factor in preparation for mating. The binding of extracellular signal molecules to either cell-surface receptors or intracellular receptors. Most signal molecules are hydrophilic and are therefore unable to cross the plasma membrane directly; instead, they bind to cell-surface receptors, which in turn generate one or more signals inside the target cell. Some small signal molecules, by contrast, diffuse across the plasma membrane and bind to receptors inside the target cell either in the cytosol or in the nucleus (as shown here). Many of these small signal molecules are hydrophobic and nearly insoluble in aqueous solutions; they are therefore transported in the bloodstream and other extracellular fluids after binding to carrier proteins, from which they dissociate before entering the target cell. Forms of intercellular signaling. (A) Contact-dependent signaling requires cells to be in direct membrane-membrane contact. (B) Paracrine signaling depends on signals that are released into the extracellular space and act locally on neighboring cells. (C) Synaptic signaling is performed by neurons that transmit signals electrically along their axons and release neurotransmitters at synapses, which are often located far away from the cell body. (D) Endocrine signaling depends on endocrine cells, which secrete hormones into the bloodstream that are then distributed widely throughout the body. Many of the same types of signaling molecules are used in paracrine, synaptic, and endocrine signaling; the crucial differences lie in the speed and selectivity with which the signals are delivered to their targets. The contrast between endocrine and synaptic signaling. In complex animals, endocrine cells and nerve cells work together to coordinate the diverse activities of the billions of cells. Whereas different endocrine cells must use different hormones to communicate specifically with their target cells, different nerve cells can use the same neurotransmitter and still communicate in a highly specific manner. (A) Endocrine cells secrete hormones into the blood, which signal only the specific target cells that recognize them. These target cells have receptors for binding a specific hormone, which the cells “pull” from the extracellular fluid. (B) In synaptic signaling, by contrast, specificity arises from the synaptic contacts between a nerve cell and the specific target cells it signals. Usually, only a target cell that is in synaptic communication with a nerve cell is exposed to the neurotransmitter released from the nerve terminal (although some neurotransmitters act in a paracrine mode, serving as local mediators that influence multiple target cells in the area). An animal cell's dependence on multiple extracellular signals. Each cell type displays a set of receptors that enables it to respond to a corresponding set of signal molecules produced by other cells. These signal molecules work in combinations to regulate the behaviour of the cell. As shown here, an individual cell requires multiple signals to survive (blue arrows) and additional signals to divide (red arrow) or differentiate (green arrows). If deprived of appropriate survival signals, a cell will undergo a form of cell suicide known as programmed cell death, or apoptosis. The nuclear receptor superfamily. All nuclear hormone receptors bind to DNA as either homodimers or heterodimers, but for simplicity we show them as monomers here. (A) The receptors all have a related structure. The short DNA-binding domain in each receptor is shown in green. (B) A receptor protein in its inactive state is bound to inhibitory proteins. Domain-swap experiments suggest that many of the ligandbinding, transcription-activating, and DNAbinding domains in these receptors can function as interchangeable modules. (C) The binding of ligand to the receptor causes the ligand-binding domain of the receptor to clamp shut around the ligand, the inhibitory proteins to dissociate, and coactivator proteins to bind to the receptor's transcription-activating domain, thereby increasing gene transcription. (D) The threedimensional structure of a ligand-binding domain with (right) and without (left) ligand bound. Note that the blue α helix acts as a lid that snaps shut when the ligand (shown in red) binds, trapping the ligand in place. Responses induced by the activation of a nuclear hormone receptor. (A) Early primary response and (B) delayed secondary response. The figure shows the responses to a steroid hormone, but the same principles apply for all ligands that activate this family of receptor proteins. Some of the primary-response proteins turn on secondary-response genes, whereas others turn off the primary-response genes. The actual number of primary- and secondaryresponse genes is greater than shown. As expected, drugs that inhibit protein synthesis suppress the transcription of secondary-response genes but not primary-response genes, allowing these two classes of gene transcription responses to be readily distinguished. Three classes of cell-surface receptors. (A) Ion-channel-linked receptors (B) G-protein-linked receptors (C) enzyme-linked receptors Although many enzyme-linked receptors have intrinsic enzyme activity, as shown on the left, many others rely on associated enzymes Different kinds of intracellular signaling proteins along a signaling pathway from a cell-surface receptor to the nucleus. In this example, a series of signaling proteins and small intracellular mediators relay the extracellular signal into the cell, causing a change in gene expression. The signal is amplified, altered (transduced), and distributed en route. Many of the steps can be modulated by other extracellular and intracellular signals, so that the final result of one signal depends on other factors affecting the cell. Ultimately, the signaling pathway activates (or inactivates) target proteins that alter cell behavior. In this example, the target is a gene regulatory protein. Two types of intracellular signaling proteins that act as molecular switches. In both cases, a signaling protein is activated by the addition of a phosphate group and inactivated by the removal of the phosphate. (A) The phosphate is added covalently to the signaling protein by a protein kinase. (B) A signaling protein is induced to exchange its bound GDP for GTP. To emphasize the similarity in the two mechanisms, ATP is shown as APPP, ADP as APP, GTP as GPPP, and GDP as GPP. Signal integration Extracellular signals A and B both activate a different series of protein phosphorylations, each of which leads to the phosphorylation of protein Y but at different sites on the protein. Protein Y is activated only when both of these sites are phosphorylated, and therefore it becomes active only when signals A and B are simultaneously present. For this reason, integrator proteins are sometimes called coincidence detectors. Intracellular signalling complexes enhance the speed, the efficiency, and the specificity of the response A specific signalling complex can be formed using modular interaction domains Signalling through G-protein-coupled cell-surface receptors Cyclic AMP is synthesized by the adenylyl cyclase from ATP. It is a cyclization reaction that removes two phosphates as pyrophosphate. Cyclic AMP is short-lived. It is rapidly hydrolyzed by phosphodiesterases to give 5’-AMP as shown on the figure. The pyrophosphate is hydrolyzed to inorganic phosphates. This reaction is the thermodynamic driver for the synthesis of cAMP. A cultured nerve cell responding to the neurotransmiter serotonin. Serotonin acts through a GPCR and activates cAMP synthesis. The cells express a fluorescent proteins that changes its fluorescence upon binding of cAMP. Blue indicate low concentration of cAMP, yellow – intermediate and, red a high concentration of cAMP.