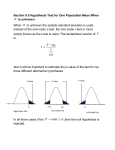
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Storage cells in the bone marrow
Heritability of IQ wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Frameshift mutation wikipedia , lookup
Behavioural genetics wikipedia , lookup
Koinophilia wikipedia , lookup
Gene expression programming wikipedia , lookup
History of genetic engineering wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Genetic engineering wikipedia , lookup
Point mutation wikipedia , lookup
Human genetic variation wikipedia , lookup
Population genetics wikipedia , lookup
Designer baby wikipedia , lookup
Medical genetics wikipedia , lookup
Genetic testing wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Public health genomics wikipedia , lookup
Oncogenomics wikipedia , lookup
Microevolution wikipedia , lookup
Acute Myeloid Leukaemia— phenotype and genotype Barbara J. Bain Department of Haematology St Mary’s Hospital, London The phenotypic diagnosis • A case of leukaemia was first clearly described in 1845 by John Hughes Bennett and another, 6 weeks later, by Rudolf Virchow • These were phenotypic diagnoses at autopsy, the blood being examined microscopically • A few years later Virchow was the first to use the term “Leukhemia” Things moved on The next one and a half centuries saw advances in the phenotypic diagnosis of leukaemia based on • Better stains and microscopes • Examination of the blood and, later, the bone marrow during life Things moved on further Further advances improved the phenotypic diagnosis. These were • Cytochemical stains • Immunophenotyping • Integration and codification of phenotypic diagnoses And further still The FAB classifications from the last quarter of the 20th century can be seen as the culmination of phenotypic diagnosis • The FAB group defined, and categorized and gave haematologists and, later, cytogeneticists throughout the world a common language • Phenotypic diagnosis had become quite sophisticated But meanwhile — Cytogenetic techniques were improving and with the introduction of chromosome banding there was a quantum leap in what could be recognized • The incorporation of cytogenetic information into leukaemia classification started, e.g. MIC Group 1986. And furthermore — Molecular genetics was invented Now we have • FISH, SKY, spectral karyotyping, CGH • PCR and RT-PCR • Microarray analysis What does this mean for leukaemia diagnosis and classification? • Can we move from a MIC to a MIC-M approach • Should we do so? The WHO classification The WHO classification has taken an approach which is partly based on cytogenetic and molecular genetic analysis • First, antecedent factors are considered • Secondly, genetic features are considered • Thirdly, phenotype is used to categorize the remaining patients The WHO classification • When appropriate, patients are first assigned to the category of therapy-related AML, regardless of whether they have recurring cytogenetic abnormalities • These cases are further categorized as – Alkylating agent-related – Topoisomerase II-interactive drug-related – Other WHO and therapy-related AML This is, in part, a genetic classification • Alkylating agent-related AML is characterized by abnormalities of chromosomes 5 and 7, inv(3), t(3;3), t(6;9), t(8;16) and complex rearrangements WHO and therapy-related AML Topoisomerase II-inhibitor-related AML is characterized by balanced translocations with 21q22 (AML1) and 11q23 (MLL) breakpoints including t(3;21), t(8;21), t(4;11), t(9;11) and t(11;19)(q23;p13.1) [but not t(11;19)(q23;p13.3)] WHO and therapy-related AML • Topoisomerase II-inhibitor-related AML is associated not only with t(8;21)(q22;q22) but also with other translocations usually found in de novo AML, e.g. t(15;17)(q22;q12) and inv(16)(p13q22) Where does therapy-related AML with recurrent cytogenetic abnormality belong? It may be important to classify such cases as therapy-related in order to accurately judge the magnitude of the problem of therapyinduced AML (and ALL) These cases might also be prognostically worse than de novo cases Where does therapy-related AML with recurrent cytogenetic abnormality belong? However—t-AML and de novo AML with the same cytogenetic abnormality have a lot of features in common and maybe the prognosis is not so very different Therapy-related AML with t(8;21)(q22;q22) Slovak et al. (2002) reported a median survival of < 3 years and a 5 year survival of < 30% However the treatment was not standardized and in the larger group of AML patients with a 21q22 bp a quarter of patients were untreated or undertreated Slovak et al. (2002) Genes Chromosomes Cancer, 33, 379. Therapy-related AML with t(15;17)(q22;q21) Andersen et al. (2002) reported, for intensively treated patients, that there was: • a 69% CR rate • a median survival of 29 months • a 5-year survival of 45% Andersen et al. (2002) Genes Chromosomes Cancer, 33, 395. Therapy-related AML with t(15;17)(q22;q21) n CR rate 34 97% ‘secondary’ 642 93% de novo 4-yr EFS 4-yr OS 65% 85% 68% 78% ‘Secondary’ = 15 surgery, 17 RT, 10 Chemo, 10 Chemo + RT Pulsoni et al. (2002) Blood, 100, 1972 Therapy-related AML with inv(16)(p13q22) Andersen et al (2002) reported , with intensive treatment: • 85% CR • 29 months median survival • 45% 5-year survival • Andersen et al. (2002) Genes Chromosomes Cancer, 33, 395. What should we do? • We should follow the WHO classification but should note the presence of relevant cytogenetic abnormalities • We should recognize that topoisomerase IIinhibitor-related secondary AML is quite a different matter from t-AML related to alkylating agents WHO classification AML — with recurrent cytogenetic abnormalities Advantages • Certain cytogenetic/molecular genetic categories are recognized • AML, e.g. with t(8;21) and inv(16) with less than 30% blasts is recognized WHO classification AML — with recurrent cytogenetic abnormalities Disadvantages • Acute promyelocytic leukaemia with classical and variant translocations are lumped together • All cases of AML with all 11q23 breakpoint and MLL rearrangement are lumped together Acute promyelocytic leukaemia and “variants” (i) Subtype M3/M3v/t(15;17) M3-like/t(11;17) (q23;q21) M3-like/t(11;17) (q13;q21) M3-like/t(1;17) Gene ATRA response PML-RARA Yes PLZF-RARA No PML distrib Abnormal Normal NuMA-RARA Probably Normal NPM-RARA Normal Probably Acute promyelocytic leukaemia and “variants” (ii) RARA and the normal cell RAR binds to RXR. In the absence of RA, RAR-RXR binds to RARE in the promoter of target genes, interacts with co-repressors, attracts HDACs, leading to chromatin condensation and repression of transcription In the presence of RA, RAR-RXR is converted from a repressor to an activator since it now binds to co-activators and fails to bind to co-repressors Acute promyelocytic leukaemia and “variants” (iii) RARA and the promyelocytic leukaemia cell • PML-RAR binds more strongly to co-repressors and does not dissociate in the presence of physiological levels of RA • However, pharmacological levels of ATRA correct the situation • HDAC inhibitors should enhance the action of ATRA and appear to do so Jones and Saha (2002) Br J Haematol, 118, 714 Acute promyelocytic leukaemia and “variants” (iv) RARA and the leukaemia cell of M3-like/t(15;17)/PLZFRARA AML • PLZF is a transfer factor that binds to co-repressors • PLZF-RAR therefore binds to corepressors through two binding sites —dependent on RARA and independent of it • Pharmacological doses of ATRA don’t break the second bond and therefore cases are refractory to this therapy • However HDAC inhibitors should enhance and appear to do so Acute promyelocytic leukaemia and “variants” (v) Conclusion • Not all acute leukaemias involving the RARA gene represent the same disease – Some respond to ATRA and some do not – Some respond to arsenic trioxide and some do not – A molecular classification can help us to predict or understand response to therapy Acute myeloid leukaemia with 11q23 breakpoints and MLL rearrangement Is this one disease or many? The results of the European 11q23 workshop and other published data suggest that this is many diseases Acute myeloid leukaemia with 11q23/MLL rearrangement t(4;11) t(6;11) t(9;11) t(10;11) t(11;19)/MLL-ELL t(11;19)/MLL-ENL n 183 30 125 20 21 32 <1y 34 % 7% 17 % 32 % 14 % 41 % ALL 95 % 10 % 7% 20 % nil 66 % Acute myeloid leukaemia with 11q23/MLL rearrangement t(4;11) t(6;11) t(9;11) t(10;11) t(11;19)/MLL-ELL t(11;19)/MLL-ENL FAB M4 M4/M5a M5a M5a M4 M4/M5a t-AML/MDS 5.5 % nil 10 % 1% 7% nil Acute myeloid leukaemia with 11q23/MLL rearrangement Conclusion • Acute leukaemia with 11q23/MLL involvement is many disease not one • This does not yet have much therapeutic significance but maybe it will have in the future Are there other cytogenetic/ molecular genetic categories of AML we should recognize? M1/t(9;22)/BCR-ABL fusion • FAB: M1 (occasionally M0, M2, M4) • Usually no Auer rods • Mainly de novo but occasionally after topoisomerase-II-interactive drugs • Poor prognosis Are there other cytogenetic/ molecular genetic categories of AML we should recognize? M2 or M4Baso/t(6;9)/DEK-CAN fusion • FAB: M2 (occasionally M4 or M1) • May show basophilic differentiation and Auer rods • Mainly de novo but occasionally after topoisomerase-II-interactive drugs or alkylating agents • Poor prognosis Are there other cytogenetic/ molecular genetic categories of AML we should recognize? M5/t(8;16)/MOZ-CBP fusion • FAB: M5 or M4 with haemophagocytosis • Abnormal coagulation • de novo or secondary (topoisomerase-IIinteractive drugs or alkylating agents) • Poor prognosis Are there other cytogenetic/ molecular genetic categories of AML we should recognize? M7/t(1;22)/OTT-MAL fusion • FAB: M7 • Mainly infants Are there other cytogenetic/ molecular genetic categories of AML we should recognize? Various/inv(3) or t(3;3)/EVI1 dysregulation • Any category except M3; M7 over-represented • Platelet count often normal or even increased • Trilineage myelodysplasia • De novo, secondary to alkylating agents or transformation of a MPD Can we recognize any purely molecular categories of AML? Can we recognize any purely molecular categories of AML? Yes AML with CEBPA mutations (i) Mutations in the CEBPA gene, encoding the CCAAT/enhancer binding protein have now been identified in 7-10% of AML CEBP is exclusively expressed in myelomonocytic cells and is essential for neutrophilic differentiation AML with CEBPA mutations (ii) CEBP negatively regulates MYC and positively regulates the genes encoding the receptors for M-CSF, G-CSF and GM-CSF CEBP-deficient mice have granulopoiesis arrested at the myeloblast stage AML with CEBPA mutations (iii) CEBP mutations were found in 15 of 134 patients studied prospectively and were an independent good prognostic feature Such mutations, in the absence of FLT3 ITD, could lead to a patient being classified and treated as ‘good prognosis’ Preudhomme et al (2002) Blood, 100, 2717 What does the future hold? Is the future micro-arrays? What does the future hold? • Clinical assessment and morphology will remain crucial, particularly for diagnosis • Cytochemistry will continue to decline in importance, but should not be neglected • Immunophenotyping will hang on • Molecular diagnosis will lead to more accurate classification and scientificallybased more effective treatment