* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download allyl cysteine sulphoxide
Paracrine signalling wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biochemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metabolomics wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
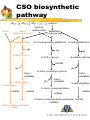
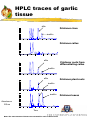
The Biochemical Synthesis of ‘Alliin’ by Garlic Jill Hughes School of Biological Sciences University of Liverpool Hamish Collin, Brian Tomsett, Meriel Jones, Rick Cosstick, Angela Tregova, and Gloria Van der Werff What is Alliin? O CH2=CHCH2SCH2CH(COOH)(NH2) (+)-S-2-Propenyl-L-cysteine S-oxide also called alliin, allyl cysteine sulphoxide or allyl CSO Alliin is a three carbon allyl group linked to the oxidised sulphur atom of the amino acid cysteine Our aim is to find out where these carbon skeletons and the sulphur originate! What is Alliin? Alliin is one member of a group of related flavour precursors - the S -alk(en)yl-L-cysteine sulphoxides O RSCH2CH(COOH)(NH2) These cysteine sulphoxides are present in varying amounts and proportions in different Allium species R = generalised alk(en)yl group The four common CSOs in Allium sp. O Alliin CH2=CHCH2SCH2CH(COOH)(NH2) and S-(E)-1-Propenyl-L-cysteine S-oxide (also called propenyl cysteine sulphoxide, propenyl CSO or isoalliin) O CH2CH=CH2SCH2CH(COOH)(NH2) S-Propyl-L-cysteine S-oxide (also called propyl cysteine sulphoxide, propyl CSO or propiin) O CH2CHCH2SCH2CH(COOH)(NH2) S-Methyl-L-cysteine S-oxide (also called methyl cysteine sulphoxide, methylCSO or methiin) O CH3SCH2CH(COOH)(NH2) Alliinase When garlic tissue is damaged, the flavour precursors are brought into contact with the enzyme Alliinase Alliinase is a C-S lyase and breaks the bond within the cysteine moiety O 2CH2=CHCH2SCH2CH(COOH)(NH2) alliinase O CH2=CHCH2S SCH2 CH= CH2 alliicin 2-propenyl-2-propenethiosulphinate pyruvate + 2 CH3(CO)(COOH) Alliinase alliicin (and its breakdown products) are responsible for the odour of freshly crushed garlic and the health giving properties Other cysteine sulphoxides are lysed by alliinase to give their respective volatiles. O RSCH2CH(COOH)(NH2) alliinase + RS=O R1S-S-R2 etc. CH3(CO)(COOH) CSO biosynthetic pathway SO42serine SO32- SO22- cysteine valine & methacrylate Allyl-S (unknown sources) S-allylglutathione glutathione (γ-glu-cys-gly) S-(2-carboxypropyl)-glutathione S-methylglutathione gly S-allyl-γ-glu-cys gly S-2-CP-γ-glu-cys gly S-methyl-γ-glu-cys HCOOH glu glu transpeptidase S-allylcysteine S-trans-1-propenyl-γ-glu-cys glu transpeptidase S-allylcysteine transpeptidase S-methylcysteine S-trans-1-propenylcysteine oxidase oxidase alliin S-allyl-cysteine sulphoxide (alliin) oxidase oxidase S-trans-1-propenylcysteine sulphoxide methiin (isoalliin) What is known already? In 1989, Jane Lancaster and her team fed labelled sulphate to cut onion leaves. From her results, she proposed that the cysteine sulphoxides were made by conjugation of the alk(en)yl moiety ( R ) to glutathione. A gamma-glutamyl, cysteine, glycine peptide RSCH2CHCONHCH2COOH NH2 CO R- cys-gly R-C-G l CH2 glu E CH2 CH(NH )(COOH) How to study a metabolic pathway Alliin and other CSOs are secondary metabolites. Non-essential for cell function but evolved with a selective advantage What is the first committed step? Is this linked to sulphur availability, as with onions, stage of development or tissue type regulated? Is the pathway controlled at the transcription, post-transcription or translation level? Where in the cell does this take place? Are gamma-glutamyl peptides compartmentalised? Can we separate Alliinase containing cells from Alliin synthesising cells? What are the metabolites of this biosynthetic pathway? How do we look at a complex network of interacting pathways without perturbing the system Initial HPLC approach to identify CSOs and possible intermediates Summary of preparative work Solvent extraction methods (based on amino acid extraction methods) and HPLC have been developed previously in this laboratory and used to estimate cysteine sulphoxides in onion (Allium cepa). These methods have been further developed and improved for larger scale analysis of garlic extracts. Method Tissue is extracted overnight in 12:5:3 Methanol:Chloroform:Water After addition of an equal volume of 9:11 Chloroform:Water, the aqueous extract is freeze dried, 50l is applied to a Phenomenex MAX-RP HPLC column with 0.03M HCl mobile phase run at 0.9ml/min@RT Standards for HPLC As many as possible: Synthesised and confirmed by NMR and mass spectrometry Purchased - amino acids, glutathione, gamma glutamyl cysteine Gifts - gamma glutamyl allyl cysteine (Thomas Haffner) Modified - oxidation of CPC and gamma glutamyl cysteine Synthesis of standards Alliin synthesised from Allyl cysteine made in the laboratory from cysteine and Allyl bromide (method based of Stoll and Seebeck, 1949) Propyl cysteine sulphoxide and n-Butyl cysteine sulphoxide similarly synthesised from Propyl cysteine (made in the laboratory from cysteine and 1-bromo-propane) and n-Butyl cysteine (made in the laboratory from cysteine and 1-bromo-butane) Methyl cysteine sulphoxide and Ethyl cysteine sulphoxide synthesised by oxidation of methyl cysteine and ethyl cysteine (Sigma chemicals) respectively Carboxy-propyl cysteine (CPC) synthesised from cysteine and methacrylic acid by a method based on that described by Schoberl, 1947 and Schoberl and Wagner, 1960 Propenyl cysteine sulphoxide Synthesised by oxidation of propenyl cysteine. O CH2CH=CH2SCH2CH(COOH)(NH2) Propenyl cysteine was synthesised by ‘base isomerisation’ with tertiary butoxide of allyl cysteine by a method based on that described by Carson and Wong(1963). This method is described for the production of cis- propenyl cysteine sulphoxide, however it should theoretically to produce both ‘cis’ and ‘trans’ isomers. It was decided to search the reaction products for the biological ‘trans’ isomer. This was successful and this synthetic method has been used, together with repeated preparative HPLC, to purify (+)-S-1-E- propenyl-Lcysteine sulphoxide from the reaction products. Confirmation of structure has been made by NMR and Mass Spectroscopy and comparison with synthetic alliin and both onion and garlic extracts (HPLC). Synthesis of standards The structure and purity of synthesised compounds have been confirmed by NMR and Mass Spectroscopy. The chemical synthetic methods described will enable future synthesis of these and similar compounds using isotopically labelled starting material Retention times of standard compounds 0.03M HCl, 0.9ml/min Serine 3.03 Methyl cysteine sulphoxide 3.28 Glutamic acid 3.53 Cysteine 3.58 Ethyl cysteine sulphoxide 3.94 Methyl cysteine 4.64 Allyl cysteine sulphoxide 4.92 CPC'oxidised' 5.29 & 5.54 Propenyl cysteine sulphoxide 5.75 Propyl cysteine sulphoxide 6.4 Valine 6.43 Glutathione 7.1 Gamma glutamyl cysteine 8.45 Gamma glutamyl allyl cysteine'oxidised' 8.61 &10.08 Ethyl cysteine 8.79 n-Butyl cysteine sulphoxide 14.95 & 15.69 Allyl cysteine 15.16 CPC'oxidised' 15.89 Propenyl cysteine 19.51 & 20.8 Propyl cysteine 25.16 Gamma glutamyl allyl cysteine 44 Garlic tissue analysis The HPLC flavour precursor profiles of various Allium species and garlic tissue types have been produced. Alliin is present in garlic leaf, bulb and roots but is not observed in significant levels in undifferentiated garlic callus Upon differentiation of the callus, the new roots start to produce alliin. Precursor feeding experiments 1 Based on ‘precursor feeding to onion callus’ experiments by Selby et.al. We are starting with garlic callus Garlic callus (variety ‘Printanor’) is now routinely cultured in this laboratory and available in sufficient quantities for precursor feeding experiments. There is little background interference in undifferentiated callus It is relatively easy to introduce the substrate HPLC traces of garlic tissue alliin Printanor clove 1 0 0 8 0 6 0 isoalliin 4 0 2 0 0 2. 00 4. 00 6. 00 8. 00 1 0 0 Printanor callus 8 0 6 0 4 0 2 0 0 0. 00 2. 00 4. 00 6. 00 8. 00 alliin Printanor roots from differentiating callus 8 0 6 0 isoalliin 4 0 2 0 0 0. 00 2. 00 4. 00 6. 00 8. 00 alliin alliin 1 0 0 Printanor plant roots 8 0 isoalliin 6 0 4 0 2 0 0 0. 00 2. 00 4. 00 alliin 6. 00 8. 00 1 0 0 isoalliin 8 0 6 0 Absorbance 215nm 4 0 2 0 0 2 . 0 0 4 . 0 0 Time 6 . 0 0 8 . 0 0 Note: The same amount of tissue was extracted for each of these traces Printanor leaves Concentration of flavour precursors in garlic tissue Tissue Precursor concentration (mM) Alliin Isoalliin Clove 50 3 Callus 1 not detectable Callus root 3 1 Root 5 not detectable Leaf 50 10 Precursor feeding experiments 2 In initial experiments both undifferentiated and differentiating callus have been maintained for up to 15 days on a phytogel/MS medium, with and without sulphate, containing a range of potential precursors to the synthesis of Alliin (at different concentrations). This method of substrate feeding will only give a positive result if: the substrate gets into the cell enzymes are present that utilise the substrate the product is not further metabolised So far we have shown that both Allyl cysteine and Allyl thiol can be taken up by callus and converted to Alliin Precursor feeding experiments 3 This work is in progress It is possible to extend these preliminary experiments to other tissues using labelled precursors and linking HPLC to Mass Spectroscopy.