* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Keratin
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
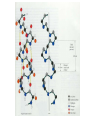
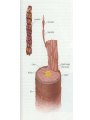
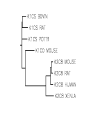
Protein Presentation: Keratin Group: Mike Dibley Chris Geiger Noel Barkhooy Keratin • Fibrous (structural) proteins. • Individual molecules combine to form insoluble structures. • Keratins are the molecular basis for hair, nails, wool, feathers, beaks, claws and horns. • Keratin is also found in the cytoskeleton of cells. • Keratin clusters are found on chromosomes 12 and 17. • alpha (cysteine rich) isomer found in cytoskeleton and hair. • beta (cysteine poor) isomer found mostly in birds and reptiles. It is the building block of scales, feathers and claws. It is rich in residues with small side chains: glycine, alanine and serine. • alpha form can be stretched up to 120% in moist heat. beta form is rigid. • Cysteine can form disulfide bridges with other cysteine residues. These cross-linkages decrease the elasticity of alpha-keratin. • In the early 1950s Linus Pauling and R.B. Corey in proposed several structures for keratin. • Observed shorter than expected amide C-N bond. They deduced that the peptide bond was planar. • A planar peptide bond reduced the number of conformations of a poly-peptide chain and led to their proposal of the alpha helix and the beta sheet. • alpha-helix explained the x-ray data which showed a repeat unit of 0.50 – 0.55 nm. This distance corresponds to the height of the rise per revolution of helix. • alpha-helix also explained a repeat unit of 0.15 nm. This distance corresponds to the height of the rise per residue. The ratio of these two numbers give the number of amino acids per revolution: 3.6 • Hydrogen bonding occurs between carbonyl oxygen and the amide hydrogen on next twist of helix. • There are many forms of alpha-keratin. • Individual alpha-keratin molecules all follow the same pattern: head, rod and tail (100:300:100). • The rod section contains 3 individual alpha-helices with short interleaving sections separating them. • Type I (40-55 kDa) are acidic. (k9-k20) • Type II (56-70 kDa) are neutral or basic. (k1-k8) • Many of the subclasses contain allelic versions (there are 6 forms of k6 (human)). • Two type I and two type II molecules form a heterotetramer. The arrangement of this molecule is a left-handed coil. Cytokeratin 12, type I Cytokeratin 3, type II Helical Structure Continued • In a coil group of 7 residues, 1st & 4th positions contain hydrophobic aa’s • These nonpolar aa’s on different helical chains attract each other and make up the inside positions of the double coils • These hydrophobic reactions stabilize the coil structure • The outside positions are mostly polar aa’s Homology • Type I & II keratins actually share a low homology; <30% at nuc. Acid level • High homology between same type keratins ~60-90% • Suggests a major sequence divergence • So although the aa sequences may differ significantly the structures the proteins take on is always the same “coiled coil” and interact with each other The End