* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cellular Respiration 2016
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
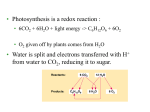
Cellular Respiration Breathe in… breathe out… or not! Boehm 2016 Introduction • Living is work. • To perform their many tasks, cells require energy from outside sources. • In most ecosystems, energy enters as sunlight. • Light energy trapped in organic molecules (such as glucose) is available to both photosynthetic organisms (autotrophs) and others that eat them (heterotrophs). Fig. 9.1 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Chloroplast Chloroplast • Chloroplasts, found in plants and eukaryotic algae, are the site of photosynthesis. • They convert solar energy to chemical energy and synthesize new organic compounds from CO2 and H2O. • Contains chlorophyll, which causes the green color of many plants. • Has small quantities of DNA that help make own proteins. Mitochondria Mitochondria • Mitochondria is the organelle that converts energy to forms that cells can use for work“powerhouse”. • Mitochondria are the sites of cellular respiration, generating ATP from the catabolism of sugars, fats, and other fuels in the presence of oxygen. • Has small quantities of DNA that help make own proteins. • The DNA is passed on from mother to childmaternity testing possible. Respiration involves glycolysis, the Krebs cycle, and electron transport: an overview • Respiration occurs in three metabolic stages: 1. glycolysis, 2. the Krebs cycle 3. the electron transport chain (oxidative phosphorylation). • NET 36 ATP formed • Total 38 ATP formed Fig. 9.6 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • ATP (adenosine triphosphate) is a type of nucleotide consisting of the nitrogenous base adenine, the sugar ribose, and a chain of three phosphate groups. Fig. 6.8a Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • The bonds between phosphate groups can be broken by hydrolysis. • Hydrolysis of the end phosphate group forms adenosine diphosphate [ATP -> ADP + Pi] and releases energy. • ATP is a renewable resource that is continually regenerated by adding a phosphate group to ADP (the reaction can go forward and reverse). Fig. 6.8b Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 9.16 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Reflect and Review • Why does a cell need ATP? • Where does glycolysis happen in a cell? • Where does the rest of CR happen in a cell? Glycolysis • Glycolysis occurs in the cytoplasm. • Breaks glucose into two molecules of pyruvate. • Happens WITHOUT oxygen! • Some ATP is generated in glycolysis by substrate-level phosphorylation. • Here an enzyme transfers a phosphate group from an organic molecule (the substrate) to ADP, forming ATP. Fig. 9.7 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • In the energy investment phase, ATP provides activation energy by phosphorylating glucose (phosphorylation makes a molecule less stable- easier to break apart). • This requires 2 ATP per glucose. • In the energy payoff phase, ATP is produced by substrate-level phosphorylation and NAD+ is reduced to NADH. • 2 ATP (net) and 2 NADH are produced per glucose. Fig. 9.8 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 9.9a Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 9.9b Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Fermentation enables some cells to produce ATP without the help of oxygen • Glycolysis generates 2 ATP whether oxygen is present (aerobic) or not (anaerobic). • Fermentation can generate ATP from glucose as long as there is a supply of NAD+ to accept electrons. • If the NAD+ pool is exhausted, glycolysis shuts down. • Under aerobic conditions, NADH transfers its electrons to the electron transfer chain, recycling NAD+. • Under anaerobic conditions, and depending on the type of organism, either lactic acid or alcoholic fermentation will occur to produce ATP. Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • In alcohol fermentation, pyruvate is converted to ethanol in two steps. • First, pyruvate is converted to a two-carbon compound, acetaldehyde by the removal of CO2. • Second, acetaldehyde is reduced by NADH to ethanol. • Alcohol fermentation by yeast is used in brewing and winemaking. Fig. 9.17a Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • During lactic acid fermentation, pyruvate is reduced directly by NADH to form lactate (ionized form of lactic acid). • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt. • Muscle cells switch from aerobic respiration to lactic acid fermentation to generate ATP when O2 is scarce. • The waste product, lactate, may cause muscle fatigue, but ultimately it is converted back to pyruvate in the liver. Fig. 9.17b Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • Some organisms (facultative anaerobes), including yeast and many bacteria, can survive using either fermentation or respiration. • At a cellular level, human muscle cells can behave as facultative anaerobes, but nerve cells cannot. • For facultative anaerobes, pyruvate can lead to either anaerobic or aerobic respiration. Fig. 9.18 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Aerobic Respiration: The Krebs cycle completes the energy-yielding oxidation of organic molecules: a closer look • More than three quarters of the original energy in glucose is still present in two molecules of pyruvate. • If oxygen is present, pyruvate enters the mitochondrion where enzymes of the Krebs cycle complete the oxidation of the organic fuel to carbon dioxide and the energy stored in NADH can be converted to ATP . Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • The conversion of pyruvate and the Krebs cycle produces large quantities of electron carriers. • Each cycle produces one ATP, three NADH, and one FADH2 (another electron carrier) per acetyl CoA. • Happens in the matrix Fig. 9.12 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings http://www.citruscollege.edu/lc/biology/PublishingImages/c07_07.jpg Electron transport chain ATP synthesis: a closer look • The vast majority of the ATP (34) comes from the energy in the electrons carried by NADH (and FADH2). • The energy in these electrons is used in the electron transport system to power ATP synthesis. • Thousands of the electron transport chain are found in the extensive surface of the inner membrane of the mitochondrion (cristae). Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 9.15 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • A protein complex, ATP synthase, in the cristae actually makes ATP from ADP and Pi. • ATP used the energy of an existing proton gradient to power ATP synthesis. • This proton gradient develops between the intermembrane space and the matrix. Fig. 9.14 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • Carbohydrates, fats, and proteins can all be catabolized through the same pathways. Fig. 9.19 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 9.16 Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings Reflect and review • Compare aerobic and anaerobic respiration. What are the similarities and differences? Is one better than the other? • Where is the most ATP made in aerobic respiration? Why is so much ATP made there? • Compare and contrast the electron transport chains from photosynthesis and cell respiration. Discuss similarities and differences.