* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Information
Survey
Document related concepts
Transcript
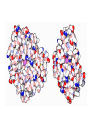
3D mRNA Function • • • • Single stranded Internal base paring and loop formation Termination of protein synthesis Balances of multi protein units pre-mRNA • The first (primary) transcript from a protein coding gene • contains both introns and exons • Pre-mRNA requires splicing (removal) of introns • the final mRNA molecule containing only exons. 3D structure of intron 3 in human cytochrome P450 2D6 structure • The 5' end of the intron is located at the top right part (white) G • The 3' end is located on the left of the figure (purple) G secondary structure • The intron is colored blue in the secondary structure. • The light red segments corresponds to the exonic subsequences. • The 5' end of intron 3 is labeled "GU“ • The 3 ' end is labeled "AG". mRNA Secondary structure Hairpin structure Hairpin structure Antibiotic resistance Bacteria are quick to fight back • Bacteria reproduce very quickly, so bacterial evolution is very fast. • Some change the drug-binding proteins in subtle ways, so that they still perform their function but do not bind to the drugs. • Some develop more effective ways to shield the sensitive enzymes from the drug or methods to pump drugs quickly away from the cell. • The most common method is to create a special enzyme, (for example: a beta-lactamase) that seeks out the drug and destroys it. Example: Penicillin-binding Proteins • The penicillin-binding proteins, (PDB entry 3pte), use a serine amino acid in their reaction, colored purple here. The serine forms a covalent bond with a peptidoglycan chain, then releases it as it forms the crosslink with another part of the peptidoglycan network. Penicillin binds to this serine but does not release it, thus permanently blocking the active site. • Other beta-lactamases do the same thing, but use a zinc ion instead of a serine amino acid to inactivate the penicillin. • Many beta-lactamases use the same machinery as used by the penicillinbinding proteins--so similar, in fact, than many researchers believe that the betalactamases were actually developed by evolutionary modification of penicillinbinding proteins. Prions – protein nature disease? • How do the prions replicate? • How do they course different diseases within one species ? • Prion protein was not modified in vitro. • Different area of brain is affected like in viral diseases. • Heat and chemicals do not inhibit the infectivity….. Nature of science • The protein-only hypothesis breaks new conceptual ground. • Those who have worked in this field under other paradigms (like the virus or virino hypotheses) are reluctant to accept this new paradigm. Nature of science • Scientists from other fields are more receptive to this hypothesis (broad support). • This hypothesis best explains all of the observations about these agents and the diseases they cause. • If it fails to do so, the hypothesis will need to be revised or rejected in favor of a better hypothesis. That is the nature of science