* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Alkaptonuria and Aspergillus nidulans
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genetic engineering wikipedia , lookup
Proteolysis wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Point mutation wikipedia , lookup
Genetic code wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Biochemistry wikipedia , lookup
Biochemical cascade wikipedia , lookup
Paracrine signalling wikipedia , lookup
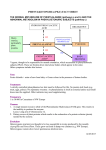
The use of Aspergillus nidulans to identify the genetic defect responsible for the human metabolic disorders Alkaptonuria and Type I Tyrosinemia. A slide set provided by Prof Miguel A Penalva Centro de Investigaciones Biologicas del CSIC, Madrid 28006, Spain. Aspergillus nidulans –a useful genetic model. Alkaptonuria is a rare genetic disorder. The discovery of the gene responsible for alkaptonuria and a number of genes responsible for human inborn errors of metabolism, have exploited the mold Aspergillus nidulans. This fungus has the experimental advantages of: • rapid growth • easy genetic manipulation • growth requirements that are readily manipulated • this filamentous fungi happens to share the same pathway of aromatic amino acid metabolism as humans, lending itself exquisitely to their study. Symptoms of alkaptonuria Normal urine Urine from patients with alkaptonuria Patients may display painless bluish darkening of the outer ears, nose and whites of the eyes. Longer term arthritis often occurs. Homogentisic acid is an intermediate in the degradation pathway of phenylalanine. The reaction is catalysed by homogentisate dioxygenase (HGO). homogentisic acid OH HOOC CH OH HGO CH2 CH O O C C CH2 CH2 COOH maleylacetoacetic acid COOH A deficiency of HGO causes alkaptonuria. Catabolic pathway for phenylalanine and tyrosine Defect here causes alkaptonuria Defect here causes Type I Tyrosinemia Homogentisate dioxygenase Fumarylacetoacetate hydrolase Characterisation of genes of the phenylalanine/ PhAc degradation pathway Genes encoding catabolic pathway enzymes are strongly induced by the pathway substrate(s), facilitating their cloning by subtractive cDNA hybridization. No transcripts were detected when tissue is grown in glucose only. Common cDNA's are subtracted out from the target population by hybridisation to RNA from tissue grown in the absence of phenylalanine, the target cDNA is thus enriched for the transcript which has been induced by the presence of its substrate phenylalanine. Characterisation of genes of the phenylalanine/PhAc degradation pathway Using a subtractional cDNA cloning strategy for A.nidulans to obtain phenylalanine degradation genes and subsequently their derived protein sequences, it was possible to screen the human databases in search of cDNAs of human homologues. The first fungal gene obtained was fahA encoding a protein FAAH (fumarylacetoacetate hydrolase). Screening showed a 47% homology with human FAAH. This enzyme catalyses the last step in catabolism of phe and tyr. 47 % identity This was significant evidence to imply that fungi and humans share the same catabolic pathway for the breakdown of phe and tyr aromatic rings. homogentisic acid C C CH2 CH2 CH COOH O O = CH O = HOOC O FAAH catalyses the last step in the degradation path of phenylalanine and tyrosine. HOOC - CH2 - CH2 - C - CH2 - C - CH2 - COOH maleylacetoacetic acid succinylacetoacetic acid spontaneous HOOC CH CH2 CH2 CH O O fumarylacetoacetate FAAH O O = C = C COOH COOH HOOC - CH2 - CH2 - C - CH2 - C - CH3 Succinylacetone toxic and mutagenic Fumarate + acetoacetate Deficiency of the enzyme FAAH results in Type I tyrosinemia Tyrosinemia is diagnosed by a blood and urine test. Tyrosinemia is treated by a low protein diet (low in phenylalanine, methionine and tyrosine) and a drug called NTCB. WHAT ARE THE SYMPTOMS OF TYROSINEMIA? The clinical features of the disease ten to fall into two categories, acute and chronic. In the so-called acute form of the disease, abnormalities appear in the first month of life. Babies may show poor weight gain, an enlarged liver and spleen, a distended abdomen, swelling of the legs, and an increased tendency to bleeding, particularly nose bleeds. Jaundice may or may not be prominent. Despite vigorous therapy, death from hepatic failure frequently occurs between three and nine months of age unless a liver transplantation is performed. But the search for the defective enzyme responsible for alkaptonuria was still on. •In TBLASTIN searches of the human EST database the amino acid sequence of fungal HGO identified candidate cDNAs for human HGO. •Most of these ESTs, which represent a single transcript, came from liver cDNA libraries. •The human protein encoded by this transcript showed 52% homology to fungal HGO and when expressed in bacteria it gave HGO activity. A fungal mutant strain (DfahA) deficient in the enzyme FAAH was unable to grow on phenylalanine alone. When lactose was also present the fungus strain still did not grow DfahA fahA+ Lactose phenylalanine No colony growth in mutant strain of A.nidulans Lactose +phenylalanine Then further colonies of the FAAH deficient strain developed which had resistance to the toxic phenylalanine. It was thought that other enzyme mutations upstream from FAAH were responsible. Wild type Phe Tyr DfahA DfahA hmgA- pHPP HGA lactose Lac + Phe Phe MAA FAA The double mutant colonies were able to grow in the presence of F + AcAc phenylalanine and secreted a brown pigment identified as homogentisate. Accumulation of ochronotic pigment in the culture supernatant of the ‘alkaptonuric’ fungus Alkaptonuria enjoys the historic distinction of being one of the conditions for which recessive inheritance was first proposed. This proposal was made in 1902 by the English physician Archibald Garrod. In a series of brilliant lectures in 1908 Garrod set forth the charter group of what he called "inborn errors of metabolism." The 4 conditions he labelled as inborn errors were albinism, cystinuria, pentosuria and, of course, alkaptonuria. These slides were adapted from material kindly provided by Miguel A Penalva, Centro de Investigaciones Biologicas del CSIC, Madrid 28006, Spain. With acknowedgement of the following people; Santiago Rodríguez de Córdoba Magdalena Ugarte David Timm José M. Fernández Cañón José M. Rodríguez Rodríguez