* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hemoglobin Lecture 2
Western blot wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Point mutation wikipedia , lookup
Interactome wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Epitranscriptome wikipedia , lookup
Vesicular monoamine transporter wikipedia , lookup
Signal transduction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
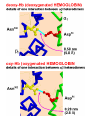
• Hemoglobin Structure – Hemoglobin is tetrameric O2 transport protein found in vertebrate erythrocytes (red blood cells) » Hb has changing X2Y2 composition over life • Always has 2 pairs of polypeptide chains • Hb A (adult) is a2b2 [HbA2 (2% Hb) is a2d2] • Early Embryo has z2e2 (like a and b) • Later Embryo z2e2 to a2g2 = Hb F (fetus) • a and z have 141 A.A.’s, slightly different • b, g, and d have 145 A.A.’s » Different oxygen affinities allow passing of O2 from mother to fetus (more later) – X-Ray Crystal Structure » 23 year project of Max Perutz (1959) » 4 subunits packed in tetrahedral array » One heme/subunit, near surface (25Å apart) » a contacts both b; no a—a or b—b contact » Hb subunits are similar to Mb • Only 18% of AA’s conserved; same shape • “Globin Fold” common to all vertebrates • Places Heme in correct environment to bind O2 reversibly • Conserve AA’s inclue F8 His and E7 His • Polar/Polar and Nonpolar/Nonpolar subst. In contrast to myoglobin hemoglobin has 4°structure • Allosteric Interactions of Hb O2 Binding – Allosteric Interactions = those between spatially separated parts of a protein » O2 binding is cooperative » O2 binding is affected by H+, CO2 binding and vice versa » The organic phosphate BPG regulates O2 binding – Cooperative O2 Binding of Hb » Saturation Y # occupied sites 0 1 total # of sites » Mb vs. Hb Y (Oxygen Dissociation Curves) • YMb > YHb at any pO2 (partial pressure O2) • P50 = pO2 at which Y = 50% • Mb P50 = 1 torr (1 atm = 760 torr) • Hb P50 = 26 torr •Shapes of the curves •Mb has the shape of hyperbola MbO2 K K [Mb][O 2 ] [MbO 2 ] Y Mb + O2 Y [MbO 2 ] [MbO 2 ] [Mb] [O 2 ] pO 2 [O 2 ] [K] pO 2 P50 •Hb has sigmoidal shape K Hb(O2)n Hb + nO2 pO 2 (pO 2 ) Y Y n n (pO 2 ) (P50 ) 1 - Y P50 n n myoglobin 1 SATURATION hemoglobin 0 10 50 o2O2 PRESSURE (torr) » Hill Plots Tell Us About Cooperativity Y log n log pO2 - n log P50 1- Y Y log 1- Y Mb n = 1.0 Hb n =2.8 Log (pO2) • n = Hill Coefficient indicates cooperativity • Mb: n = 1.0 = independent O2 binding • Hb: n = 2.8 = O2 cooperative binding – Binding the first O2 makes it easier to bind the next, and so on – Dissociating the first O2 makes it easier to dissociate the next one » Why is Cooperativity good in Hb? • Y changes very rapidly with pO2 • Lung pO2 = 100torr, Muscle pO2 = 20 n = 1 then Ylung = 0.79, Ymuscle = 0.43 (0.36 delivered) n = 2.8 then Ylung = 0.98, Ymuscle = 0.32 (0.66 delivered) Hb is 1.8 times as efficient as Mb Hb P50 lies between lungs and muscle – H+ and CO2 effects on Hb O2 Binding » Bohr Effect: Increased [H+] decreases binding • Mb O2 binding is not affected by [H+] • Contracting muscle generates H+ and CO2 • This helps Hb release O2 • Deoxy-Hb binds H+ stronger than oxy-Hb » The effect is mutual: high [O2] causes H+ to dissociate from Hb » CO2 effect on Hb binding –Organic Phosphate Regulation of Hb O2 binding »BPG is an organic phosphate Concentrations of glycolytic intermediates in erthyrocytes mM 5000 83 14 31 138 19 1 4000 (BPG) 118 30 23 51 2900 glucose glucose- 6- P fructose- 6- P fructose- 1,6- P dihydroxyacetone- P glyceraldehyde- 3- P 1,3 bisphosphoglycerate 2,3 bisphosphoglycerate 3 phosphoglycerate 2 phosphoglycerate phosphoenolpyruvate pyruvate lactate From S. Minakami and H. Yoshikawa. Biochem.Biophys.Res.Comm. 18(1965):345. - O O C H C O CH2 O P O - O- O - O P O O - 2,3 bisphospho-glycerate (BPG) BPG Lowers the binding affinity of Hb for O2 •[BPG] = 0, Hb P50 = 1 torr •[BPG] = 4000mM, Hb P50 = 26 torr •Without BPG, Hb couldn’t unload O2 in cells No BPG 1 SATURATION With BPG 0 10 50 o2O2 PRESSURE (torr) BPG acts by stabilizing deoxyHb BPG binds by electrostatic interactions to the highly electropositive region (red) in a crevice between the 4 subunits BPG binding site » BPG ensures that O2 can be unloaded at the peripheral tissues • by decreasing the affinity of Hb for O2 about 26 fold • increasing O2, on the other hand, promotes the formation of oxyHb whose changed conformation prevents BPG binding because the binding cavity becomes too small » Fetal Hb has a lower affinity for 2,3-BPG and therefore has a higher affinity for O2 • BPG regulates O2 binding between Hb types • This allows transfer of O2 from mother to child • This explains the need for multiple Hb types • If [BPG] = 0, HbA > HbF for O2 binding • HbF has neutral Serine in place of HbA His 1 SATURATION HbF HbA O2 flows from mom to baby ! 0 10 50 o2O2 PRESSURE (torr) – Structural Basis for Cooperativity » Interactions between subunits • A dissociated Hb subunit binds O2 like Mb • A b4 tetramer binds O2 like Mb • Cooperativity must involve subunit interactions » OxyHb and DeoxyHb have very different quaternary structures • OxyHb is more compact (bFe—bFe changes from 40 to 33Å) • When O2 binds, a—b contacts change as H-bonds are adjusted • Electrostatic bonds (Salt Links) also change: OxyHb the CO2- termini can freely rotate, DeoxyHb CO2- termini salt linked • DeoxyHb has T-form (“taut”) • OxyHb has R-form (“relaxed”) » Changes at the Heme initiate structure switch • DeoxyHb has Fe 0.3Å out of plane N N Fe2+ N N N • OxyHb has Fe in plane of porphyrin O O N 2+ N Fe N N N • Fe atom pulls the bound F8 His with it – Shifts the whole F helix, EF corner – Salt links are broken at ab interface – T-form becomes R-form – R-form has greater O2 affinity – Cooperativity set in motion • BPG stabilizes deoxyHb T-form by creating more contacts • O2 binding to Hb causes dissociation of BPG because the cavity gets too small. This favors the R-form as well. – Models for Allosteric Interactions » Sequential Model • Only T and R forms possible for each unit • T to R transition of each subunit is induced by O2 binding, but this does not change the form of other subunits • Conformational changes enhance O2 binding at the next subunit, but O2 must bind each subunit before it switches to R O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 » Concerted Model • Whole protein changes from T to R form upon initial O2 binding • O2 has higher affinity for the unbound R subunits • This explains cooperativity O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 O2 » Actual: mix of the two models. Hb is predominantly T until ~2 O2 molecules are bound, then it goes all R. myoglobin 1 SATURATION hemoglobin 0 10 50 o2O2 PRESSURE (torr) Sickle-cell anemia • A Glu normally resides at position 6 of each b- subunit. In HbS this amino is mutated to Val Glu 6 a b b a Glu 6 • the Val for Glu mutation makes deoxy-HbS insoluble -findout why! Sickle-cell anemia • the Val for Glu mutation makes deoxyHbS insoluble In deoxy-HbS, b-subunit residues Phe 85 and Leu 88 reside at the surface and bond with Val 6 on another b-subunit. This leads to the formation of long filamentous strands of deoxy-HbS and to the sickling deformation of the erthyrocytes In oxy-HbS, b-subunit residues Phe 85 and Leu 88 do not reside at the cell surface, so oxy-HbS does not aggregate. Thus, its oxygen binding capacity and allosteric properties are largely retained. Hemoglobin : a portrait of a soluble protein with 4° stucture A SUMMARY • the heme prosthetic group is tightly bound in the protein and is essential for function • steric relationships within Hb ensure that the heme group has appropriate reactivity • hemoglobin has quaternary structure which gives it unique O2 binding properties allosterism and cooperativity of binding • 2,3-bisphosphoglycerate is a regulatory molecule that stabilizes deoxy-Hb and is essential for the allosterism and cooperativity of binding in Hb • there is considerable interplay between the oxygen binding affinity of Hb and [H+], [CO2] and [2,3-BPG] • the interplay between various sites in Hb is mediated through changes in quaternary structure • Sickle-cell anemia is an example of a genetically transmitted disease which highlights the effect of one amino acid substitution on protein structure and function