* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download video slide - Green River Community College
Survey
Document related concepts
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Biochemistry wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Transcript
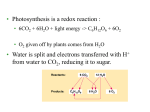
Chapter 9 Cellular Respiration: Harvesting Chemical Energy PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Overview: Life Is Work • Living cells – Require transfusions of energy from outside sources to perform their many tasks Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 1. Why do animals eat and breath? – Cells require ______________ and _______________ for growth, development, maintenance and repair 2. From what kind of food molecules does the energy come from? 3. What in these compounds provides the energy for cellular work? 4. Can this energy be used directly by cells? 5. From what kind of molecules can this energy be used directly? Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 6. How is ATP produced from the breakdown of sugars? • What is this process called? 7. What’s needed for this process to occur? 8. What is the net equation for this process under aerobic conditions? Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • The giant panda – Obtains energy for its cells by eating plants Figure 9.1 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Energy – Flows into an ecosystem as sunlight and leaves as heat Light energy ECOSYSTEM Photosynthesis in chloroplasts Organic CO2 + H2O + O2 Cellular molecules respiration in mitochondria ATP powers most cellular work Figure 9.2 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Heat energy • Concept 9.1: Catabolic pathways yield energy by oxidizing organic fuels Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Catabolic Pathways and Production of ATP • The breakdown of organic molecules is exergonic Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • One catabolic process, fermentation – Is a partial degradation of sugars that occurs without oxygen Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Cellular respiration – Is the most prevalent and efficient catabolic pathway – Consumes oxygen and organic molecules such as glucose – Yields ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • To keep working – Cells must regenerate ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Redox Reactions: Oxidation and Reduction • Catabolic pathways yield energy – Due to the transfer of electrons Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings The Principle of Redox • Redox reactions – Transfer electrons from one reactant to another by oxidation and reduction Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • In oxidation – A substance loses electrons, or is oxidized • In reduction – A substance gains electrons, or is reduced Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Examples of redox reactions becomes oxidized (loses electron) Na + Cl Na+ + becomes reduced (gains electron) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Cl– • Some redox reactions – Do not completely exchange electrons – Change the degree of electron sharing in covalent bonds Products Reactants becomes oxidized + CH4 CO 2O2 + Energy 2 H2O becomes reduced O O C O H O O H H H C + 2 H H Methane (reducing agent) Oxygen (oxidizing agent) Figure 9.3 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Carbon dioxide Water Oxidation of Organic Fuel Molecules During Cellular Respiration • During cellular respiration – Glucose is oxidized and oxygen is reduced becomes oxidized C6H12O6 + 6O2 6CO2 + 6H2O + Energy becomes reduced Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Stepwise Energy Harvest via NAD+ and the Electron Transport Chain • Cellular respiration – Oxidizes glucose in a series of steps Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Electrons from organic compounds – Are usually first transferred to NAD+, a coenzyme 2 e– + 2 H+ NAD+ Dehydrogenase O NH2 H C CH2 O O– O O P O H – O P O HO O N+ Nicotinamide (oxidized form) H OH HO CH2 N H O H HO N H OH Reduction of NAD+ + 2[H] (from food) Oxidation of NADH NH2 N N 2 e– + H+ H Figure 9.4 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings NADH H O C H N NH2 Nicotinamide (reduced form) + • NADH, the reduced form of NAD+ – Passes the electrons to the electron transport chain Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • If electron transfer is not stepwise – A large release of energy occurs – As in the reaction of hydrogen and oxygen to form water Free energy, G H2 + 1/2 O2 Figure 9.5 A Explosive release of heat and light energy H2O Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (a) Uncontrolled reaction • The electron transport chain – Passes electrons in a series of steps instead of in one explosive reaction – Uses the energy from the electron transfer to form ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 2H 1/ + 2 O2 1/ O2 (from food via NADH) Free energy, G 2 H+ + 2 e– Controlled release of energy for synthesis of ATP ATP ATP ATP 2 e– 2 H+ H2O Figure 9.5 B Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (b) Cellular respiration 2 The Stages of Cellular Respiration: A Preview • Respiration is a cumulative function of three metabolic stages – Glycolysis – The citric acid cycle – Oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Glycolysis – Breaks down glucose into two molecules of pyruvate • The citric acid cycle – Completes the breakdown of glucose Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Oxidative phosphorylation – Is driven by the electron transport chain – Generates ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • An overview of cellular respiration Electrons carried via NADH and FADH2 Electrons carried via NADH Citric acid cycle Glycolsis Pyruvate Glucose Cytosol Mitochondrion ATP Figure 9.6 Oxidative phosphorylation: electron transport and chemiosmosis Substrate-level phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings ATP Substrate-level phosphorylation ATP Oxidative phosphorylation • Both glycolysis and the citric acid cycle – Can generate ATP by substrate-level phosphorylation Enzyme Enzyme ADP P Substrate + Figure 9.7 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Product ATP • Concept 9.2: Glycolysis harvests energy by oxidizing glucose to pyruvate • Glycolysis – Means “splitting of sugar” – Breaks down glucose into pyruvate – Occurs in the cytoplasm of the cell Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Glycolysis consists of two major phases – Energy investment phase – Energy payoff phase Citric acid cycle Glycolysis Oxidative phosphorylation ATP ATP ATP Energy investment phase Glucose 2 ATP + 2 P 2 ATP used Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e- + 4 H + 4 ATP formed 2 NADH + 2 H+ 2 Pyruvate + 2 H2O Glucose 4 ATP formed – 2 ATP used Figure 9.8 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 2 NAD+ + 4 e– + 4 H + 2 Pyruvate + 2 H2O 2 ATP + 2 H+ 2 NADH • A closer look at the energy investment phase Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CH2OH HH H HO H HO OH H OH Glycolysis Glucose ATP 1 Hexokinase ADP CH2OH P HH OH OH H HO H OH Glucose-6-phosphate 2 Phosphoglucoisomerase CH2O P O CH2OH H HO HO H HO H Fructose-6-phosphate ATP 3 Phosphofructokinase ADP P O CH2 O CH2 O P HO H OH HO H Fructose1, 6-bisphosphate 4 Aldolase 5 H P O CH2 Isomerase C O C O CHOH CH2OH CH2 O P Figure 9.9 A Dihydroxyacetone phosphate Glyceraldehyde3-phosphate Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Citric Oxidative acid cycle phosphorylation • A closer look at the energy payoff phase Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 6 Triose phosphate dehydrogenase 2 NAD+ 2 Pi 2 NADH + 2 H+ 2 P O C O CHOH CH2 O P 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP O– 2 C CHOH CH2 O P 3-Phosphoglycerate 8 Phosphoglyceromutase 2 O– C O H C O P CH2OH 2-Phosphoglycerate 9 Enolase 2H O 2 2 O– C O C O P CH2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 O– C O C O Figure 9.8 B CH3 Pyruvate Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Concept 9.3: The citric acid cycle completes the energy-yielding oxidation of organic molecules • The citric acid cycle – Takes place in the matrix of the mitochondrion Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Before the citric acid cycle can begin – Pyruvate must first be converted to acetyl CoA, which links the cycle to glycolysis CYTOSOL MITOCHONDRION NAD+ NADH + H+ O– S CoA C O 2 C C O O 1 3 CH3 Pyruvate Transport protein Figure 9.10 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CH3 Acetyle CoA CO2 Coenzyme A • An overview of the citric acid cycle Pyruvate (from glycolysis, 2 molecules per glucose) Glycolysis Citric acid cycle ATP ATP Oxidative phosphorylatio n ATP CO2 CoA NADH + 3 H+ Acetyle CoA CoA CoA Citric acid cycle 2 CO2 3 NAD+ FADH2 FAD 3 NADH + 3 H+ ADP + P i ATP Figure 9.11 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • A closer look at the citric acid cycle Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Glycolysis Citric Oxidative acid phosphorylation cycle S CoA C O CH3 Acetyl CoA CoA SH O NADH + H+ C COO– COO– 1 CH2 COO– NAD+ 8 Oxaloacetate HO C COO– COO– CH2 COO– HO CH H2O CH2 CH2 2 HC COO– COO– Malate Figure CH2 HO Citrate 9.12 COO– Isocitrate COO– H2O COO– CH CO2 Citric acid cycle 7 3 NAD+ COO– Fumarate HC CH CH2 CoA SH 6 CoA SH COO– FAD CH2 CH2 COO– C O Succinate Pi S CoA GTP GDP Succinyl CoA ADP ATP Figure 9.12 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 4 C O COO– CH2 5 CH2 FADH2 COO– NAD+ NADH + H+ + H+ a-Ketoglutarate CH2 COO– NADH CO2 • Concept 9.4: During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis • NADH and FADH2 – Donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings The Pathway of Electron Transport • In the electron transport chain – Electrons from NADH and FADH2 lose energy in several steps Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • At the end of the chain – Electrons are passed to oxygen, forming water NADH 50 Free energy (G) relative to O2 (kcl/mol) FADH2 40 FMN I Fe•S Fe•S II O 30 Multiprotein complexes FAD III Cyt b Fe•S 20 Cyt c1 IV Cyt c Cyt a Cyt a3 10 0 Figure 9.13 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 2 H + + 12 O2 H2 O Chemiosmosis: The Energy-Coupling Mechanism • ATP synthase – Is the enzyme that actually makes ATP INTERMEMBRANE SPACE H+ H+ H+ H+ H+ H+ H+ A rotor within the membrane spins clockwise when H+ flows past it down the H+ gradient. A stator anchored in the membrane holds the knob stationary. H+ ADP + Pi Figure 9.14 MITOCHONDRIAL MATRIX Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings ATP A rod (for “stalk”) extending into the knob also spins, activating catalytic sites in the knob. Three catalytic sites in the stationary knob join inorganic Phosphate to ADP to make ATP. • At certain steps along the electron transport chain – Electron transfer causes protein complexes to pump H+ from the mitochondrial matrix to the intermembrane space Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • The resulting H+ gradient – Stores energy – Drives chemiosmosis in ATP synthase – Is referred to as a proton-motive force Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Chemiosmosis – Is an energy-coupling mechanism that uses energy in the form of a H+ gradient across a membrane to drive cellular work Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Chemiosmosis and the electron transport chain Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP Inner Mitochondrial membrane ATP ATP H+ H+ H+ Intermembrane space Protein complex of electron carners Q I Inner mitochondrial membrane IV III ATP synthase II FADH2 NADH+ Mitochondrial matrix H+ Cyt c FAD+ NAD+ 2 H+ + 1/2 O2 H2O ADP + (Carrying electrons from, food) ATP Pi H+ Chemiosmosis Electron transport chain + ATP synthesis powered by the flow Electron transport and pumping of protons (H ), + + which create an H gradient across the membrane Of H back across the membrane Figure 9.15 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxidative phosphorylation An Accounting of ATP Production by Cellular Respiration • During respiration, most energy flows in this sequence – Glucose to NADH to electron transport chain to proton-motive force to ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • There are three main processes in this metabolic enterprise Electron shuttles span membrane CYTOSOL MITOCHONDRION 2 NADH or 2 FADH2 2 NADH 2 NADH Glycolysis Glucose 2 Pyruvate 6 NADH Citric acid cycle 2 Acetyl CoA + 2 ATP by substrate-level phosphorylation Maximum per glucose: + 2 ATP 2 FADH2 Oxidative phosphorylation: electron transport and chemiosmosis + about 32 or 34 ATP by substrate-level by oxidative phosphorylation, depending on which shuttle transports electrons phosphorylation from NADH in cytosol About 36 or 38 ATP Figure 9.16 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • About 40% of the energy in a glucose molecule – Is transferred to ATP during cellular respiration, making approximately 38 ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Concept 9.5: Fermentation enables some cells to produce ATP without the use of oxygen • Cellular respiration – Relies on oxygen to produce ATP • In the absence of oxygen – Cells can still produce ATP through fermentation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Glycolysis – Can produce ATP with or without oxygen, in aerobic or anaerobic conditions – Couples with fermentation to produce ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Types of Fermentation • Fermentation consists of – Glycolysis plus reactions that regenerate NAD+, which can be reused by glyocolysis Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • In alcohol fermentation – Pyruvate is converted to ethanol in two steps, one of which releases CO2 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • During lactic acid fermentation – Pyruvate is reduced directly to NADH to form lactate as a waste product Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 2 ADP + 2 P1 2 ATP O– C O Glucose Glycolysis C O CH3 2 Pyruvate 2 NADH 2 NAD+ H 2 CO2 H H C OH C O CH3 CH3 2 Ethanol 2 Acetaldehyde (a) Alcohol fermentation 2 ADP + 2 Glucose P1 2 ATP Glycolysis O– C O C O O 2 NAD+ 2 NADH C O H C OH CH3 2 Lactate Figure 9.17 (b) Lactic acid fermentation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CH3 Fermentation and Cellular Respiration Compared • Both fermentation and cellular respiration – Use glycolysis to oxidize glucose and other organic fuels to pyruvate Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Fermentation and cellular respiration – Differ in their final electron acceptor • Cellular respiration – Produces more ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Pyruvate is a key juncture in catabolism Glucose CYTOSOL Pyruvate No O2 present Fermentation O2 present Cellular respiration MITOCHONDRION Ethanol or lactate Figure 9.18 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Acetyl CoA Citric acid cycle The Evolutionary Significance of Glycolysis • Glycolysis – Occurs in nearly all organisms – Probably evolved in ancient prokaryotes before there was oxygen in the atmosphere Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Concept 9.6: Glycolysis and the citric acid cycle connect to many other metabolic pathways Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings The Versatility of Catabolism • Catabolic pathways – Funnel electrons from many kinds of organic molecules into cellular respiration Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • The catabolism of various molecules from food Proteins Carbohydrates Amino acids Sugars Fats Glycerol Glycolysis Glucose Glyceraldehyde-3- P NH3 Pyruvate Acetyl CoA Citric acid cycle Figure 9.19 Oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Fatty acids Biosynthesis (Anabolic Pathways) • The body – Uses small molecules to build other substances • These small molecules – May come directly from food or through glycolysis or the citric acid cycle Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Regulation of Cellular Respiration via Feedback Mechanisms • Cellular respiration – Is controlled by allosteric enzymes at key points in glycolysis and the citric acid cycle Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • The control of cellular respiration Glucose Glycolysis Fructose-6-phosphate – Inhibits AMP Stimulates + Phosphofructokinase – Fructose-1,6-bisphosphate Inhibits Pyruvate Citrate ATP Acetyl CoA Citric acid cycle Figure 9.20 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxidative phosphorylation - Superoxide radical, O2 , formation • O2 - • O2 - generated constantly as part of normal aerobic life formed in mitochondria when O2 is reduced along the electron transport chain Oxygen Free Radical Theory of Aging • E.T.C. Superoxide Radical, O2- SOD (superoxide dismutase) converts O2- to hydrogen peroxide, H2O2 Catalase converts H2O2 to water and O2 OR • H2O2 moves to the nucleus of the cell H2O2 reacts with Fe2+ produces hydroxyl radical Damages DNA and most everything around it Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxygen Free Radical Theory of Aging • Oxygen is slowly killing us! • Raj Sohal’s (Southern Methodist University) – Has doubled or tripled the life span of house flies if he restricts there movement and hence the amount of oxygen they consume. – Gene therapy can be used to increase longevity by introducing genes that encode the enzymes the SOD (superoxide dismutase) and Catalase. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxygen Free Radical Theory of Aging 1. Vitamins C (water-soluble) and E (fatsoluble) are vitamins that deactivate free radicals. 2. Why is it most beneficial to take both of them, rather than just one or the other? Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxygen Free Radical Theory of Aging • SOD and catalase levels increase when humans exercise, thus protecting us from the extra free radicals produced as a consequence of increased oxygen consumption. • House flies do not have the genes to produce SOD and catalase consequences? Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Glucose Cross-linking with Proteins • • Diabetics – Higher than normal blood levels of glucose. – Causes diabetics to age ~one-third faster Cross-linking makes proteins less flexible – makes body parts less flexible and stiffer – major cause of aging in many tissues: • skin, bones, lungs, eyes, joints, and blood vessels. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings