* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Photosynthesis wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
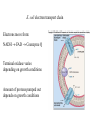
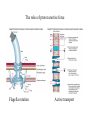
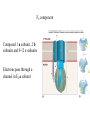
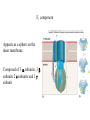
Electron transport chains Electrons move from a carrier with a lower standard reduction potentials (EO) to a carrier with a higher EO Mitochondrial electron transport chain Electrons eventually combine with 1/2 O2 and 2 H+ to form H2O Protons pumped across the membrane at various points during electron transport E. coli electron transport chain Electrons move from: NADH FAD Coenzyme Q Terminal oxidase varies depending on growth conditions Amount of protons pumped out depends on growth conditions P. denitrificans electron transport chains Has both aerobic and anaerobic electron transport chains Anaerobic chain uses NO3- as the final electron acceptor Oxidative phosphorylation Is dependent on the proton motive force and chemiosmosis The proton motive force Protons are pumped from the interior to the exterior of the membrane resulting in a gradient of protons and a membrane potential The roles of proton motive force Powers rotation of bacterial flagella Required for some types of active transport Generation of ATP The roles of proton motive force Flagella rotation Active transport Chemiosmosis Diffusion of protons back across the membrane drives the formation of ATP by ATP synthase ATP synthase Composed of 2 components: F0 - membrane embedded F1- attached to inner membrane F0 component Composed 1 a subunit, 2 b subunits and 9-12 c subunits Electrons pass through a channel in F0 a subunit F1 component Appears as a sphere on the inner membrane Composed of 3 subunits, 3 subunits 2 subunits and 1 subunit F1 component Passage of electrons through F0 causes subunit to rotate Rotation causes conformational changes in subunits that results in the synthesis of ATP F1 component Yield of ATP in eukaryotic cells 1 NADH generates 2-3 ATPs 1 FADH2 generates 2 ATPs Actual yield can be closer to 30 ATPs Yield of ATP in prokaryotic cells Prokaryotic cells generate less ATP Amounts vary depending on growth conditions Anaerobic respiration Final electron acceptor is an inorganic molecule other than oxygen Major electron acceptors are nitrate, sulfate and CO2 Metals and certain organic molecules can also be reduced Anaerobic respiration Reduction of nitrate in respiration known as dissimilatory nitrate reduction Nitrate often reduced sequentially to nitrogen gas (N2) Process referred to as denitrification Carbohydrate catabolism Glucose, fructose and mannose can enter glycolytic pathway after phosphorylation Galactose is modified before being transformed into glucose-6-P Carbohydrate catabolism Disaccharides and polysaccharides must be cleaved into monosaccharides Can be cleaved by hydrolysis or phosphorolysis (results in the addition of a phosphate group) Carbohydrate catabolism Reserve polymers like glycogen and starch are degraded by phosphorolysis to release glucose-1-P Converted to glucose-6-P and enters glycolytic pathway Poly--hydroxybutyrate converted to acetyl-CoA and enters the TCA cycle Lipid catabolism Triacylglycerides are composed of glycerol and three fatty acids Lipases separate glycerol from fatty acids Glycerol phosphorylated and converted to dihydroxyacetone phosphate glyceraldehyde3-P glycolysis Lipid catabolism Fatty acids are converted to CoA esters and oxidized by the -oxidation pathway Fatty acids degraded to acetylCoA TCA cycle Lipid catabolism Fatty acids are converted to CoA esters and oxidized by the -oxidation pathway Fatty acids degraded to acetylCoA TCA cycle -oxidation pathway Produces 1. Acetyl-CoA 2. NADH 3. FADH2 Protein and amino acid catabolism Proteases hydrolyze proteins and polypeptides into amino acids Removal of amino group referred to as deamination Deamination Usually accomplished by transamination Amino group transferred to an -keto acid acceptor Deamination Organic acid oxidized for energy or used as carbon source Deamination Excess nitrogen excreted as ammonium ion Oxidation of inorganic molecules (chemolithotrophy) Chemolithotrophs derive energy from the oxidation of inorganic molecules Most common electron donors are hydrogen, reduced nitrogen compounds, reduced sulfur compounds and ferrous iron (Fe2+) Oxygen, nitrate and sulfate can be used as the final electron acceptor Oxidation of inorganic molecules (chemolithotrophy) Hydrogen oxidation Several bacteria possess a hydrogenase enzyme that catalyzes the reaction: H2 2H+ + 2eElectrons can be donated to an electron transport chain or NAD+ Nitrogen oxidation Species of Nitrosomonas and Nitrosospira oxidize ammonia to nitrite NH4+ + 3/2 O2 NO2- + H2O + 2H+ Species of Nitrobacter and Nitrococcus oxidize nitrite to nitrate NO2- + 1/2 O2 NO3- Nitrogen oxidation Two genera working together can oxidize ammonia to nitrate NH4+ + 2 O2 NO3Process referred to as nitrification Nitrogen oxidation Proton motive force can be used to produce ATP and NADH Sulfur oxidation Some microorganisms can use reduced sulfur compounds as a source of electrons Species of Thiobacillus oxidize sulfur-containing compounds to sulfuric acid (important environmental consequences) Sulfur oxidation Can generate ATP by oxidative phosphorylation and substrate level phosphorylation Substrate level phosphorylation requires the formation of adenosine 5-phosphosulfate (APS) Oxidation of inorganic molecules Much less energy is available from the oxidation of inorganic molecules than from the oxidation of organic molecules