* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document 8195411
Survey
Document related concepts
Asymmetric hydrogenation wikipedia , lookup
Elias James Corey wikipedia , lookup
Metal carbonyl wikipedia , lookup
Kinetic resolution wikipedia , lookup
Discodermolide wikipedia , lookup
Stille reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Carbohydrate wikipedia , lookup
Aldol reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
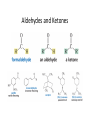
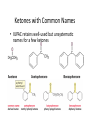
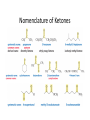
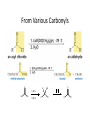
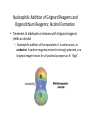
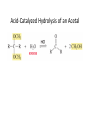
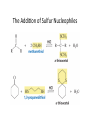
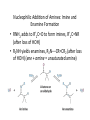
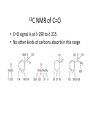
Aldehydes and Ketones Nucleophilic Addi3on Reac3ons Aldehydes and Ketones • Aldehydes (RCHO) and ketones (R2CO) are characterized by the carbonyl func3onal group (C=O) • The compounds occur widely in nature as intermediates in metabolism and biosynthesis Aldehydes and Ketones Nomenclature of Aldehydes Naming the Aldehyde as a Subs3tuent Naming Ketones • Replace the terminal -‐e of the alkane name with –one • Parent chain is the longest one that contains the ketone group – Numbering begins at the end nearer the carbonyl carbon Ketones with Common Names • IUPAC retains well-‐used but unsystema3c names for a few ketones Nomenclature of Ketones Ketones and Aldehydes as Subs3tuents • The R–C=O as a subs3tuent is an acyl group, used with the suffix -‐yl from the root of the carboxylic acid – CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl • The prefix oxo-‐ is used if other func3onal groups are present and the doubly bonded oxygen is labeled as a subs3tuent on a parent chain Reac3vity of Aldehydes and Ketones Electronic Reasoning: An aldehyde has a greater partial positive charge on its carbonyl carbon than does a ketone. • Because a hydrogen is more electron withdrawing than an alkyl group. • Aldehydes are More Reac3ve than Ketone • Ketones have greater steric crowding in their transi3on states, so they have less stable transi3on states than do aldehydes. • The carbonyl carbon of an aldehyde is more accessible to the nucleophile. Reac3vity of Carbonyl Groups Preparing Aldehydes and Ketones • From Alkenes (Ozonolysis) • From Alkynes Aldehyde from terminal alkyne via Hydrobra3on Ketone from symmetrical alkyne via Hydrobora3on and Mercury2+ catalyzed hydra3on • From Alcohols (Aldehyde and Ketone • From Arene (ketones from Friedel-‐Cra[s acyla3on) • From Carbonyl Chemistry From Various Carbonyls O H O 1) R'Li R H 2) H2O O OH R R' R R' Oxida3on of Aldehydes and Ketones • CrO3 in aqueous acid oxidizes aldehydes to carboxylic acids efficiently • Silver oxide, Ag2O, in aqueous ammonia (Tollens’ reagent) oxidizes aldehydes Ketones Oxidize with Difficulty • Undergo slow cleavage with hot, alkaline KMnO4 • C–C bond next to C=O is broken to give carboxylic acids • Reac3on is prac3cal for cleaving symmetrical ketones Oxida3on of Aldehydes and Ketones The Baeyer–Villiger Oxida3on Nucleophilic Addi3on Reac3ons of Aldehydes and Ketones • Nu-‐ approaches 75° to the plane of C=O and adds to C • A tetrahedral alkoxide ion intermediate is produced Nucleophiles • Nucleophiles can be nega3vely charged ( :Nu-‐) or neutral ( :Nu) at the reac3on site • Hydride, hydroxide, organolithium, organomagnesium, acetylide, cyanide Rela3ve Reac3vity of Aldehydes and Ketones • Aldehydes are generally more reac3ve than ketones in nucleophilic addi3on reac3ons • The transi3on state for addi3on is less crowded and lower in energy for an aldehyde (a) than for a ketone (b) • Aldehydes have one large subs3tuent bonded to the C=O: ketones have two Electrophilicity of Aldehydes and Ketones • Aldehyde C=O is more polarized than ketone C=O • As in carboca3ons, more alkyl groups stabilize + character • Ketone has more alkyl groups, stabilizing the C=O carbon induc3vely Reac3vity of Aroma3c Aldehydes • Less reac3ve in nucleophilic addi3on reac3ons than alipha3c aldehydes • Electron-‐dona3ng resonance effect of aroma3c ring makes C=O less reac3ve electrophile than the carbonyl group of an alipha3c aldehyde Hydride Addi3on • Convert C=O to CH-‐OH • LiAlH4 and NaBH4 react as donors of hydride ion • Protona3on a[er addi3on yields the alcohol Cataly3c Hydrogena3on Reduces Carbon—Oxygen Double Bonds Nucleophilic Addi3on of Grignard Reagents and Organolithium Reagents: Alcohol Forma3on • Treatment of aldehydes or ketones with Grignard reagents yields an alcohol – Nucleophilic addi3on of the equivalent of a carbon anion, or carbanion. A carbon–magnesium bond is strongly polarized, so a Grignard reagent reacts for all prac3cal purposes as R: -‐ MgX+. Mechanism of Addi3on of Grignard Reagents • Complexa3on of C=O by Mg2+, Nucleophilic addi3on of R: -‐, protona3on by dilute acid yields the neutral alcohol • Grignard addi3ons are irreversible because a carbanion is not a leaving group Nucleophilic Addi3on of HCN: Cyanohydrin Forma3on • Aldehydes and unhindered ketones react with HCN to yield cyanohydrins, RCH(OH)C≡N • Addi3on of HCN is reversible and base-‐catalyzed, genera3ng nucleophilic cyanide ion, CN-‐ • Addi3on of CN-‐ to C=O yields a tetrahedral intermediate, which is then protonated • Equilibrium favors adduct Uses of Cyanohydrins • The nitrile group (⎯C≡N) can be reduced with LiAlH4 to yield a primary amine (RCH2NH2) • Can be hydrolyzed by hot acid to yield a carboxylic acid Cataly3c Hydrogena3on Reduces Carbon—Nitrogen Double and Triple Bonds Hydra3on of Aldehydes • Aldehyde oxida3ons occur through 1,1-‐diols (“hydrates”) • Reversible addi3on of water to the carbonyl group • Aldehyde hydrate is oxidized to a carboxylic acid by usual reagents for alcohols Nucleophilic Addi3on of H2O: Hydra3on • Aldehydes and ketones react with water to yield 1,1-‐diols (geminal (gem) diols) • Hyrda3on is reversible: a gem diol can eliminate water Base-‐Catalyzed Addi3on of Water • Addi3on of water is catalyzed by both acid and base • The base-‐catalyzed hydra3on nucleophile is the hydroxide ion, which is a much stronger nucleophile than water Acid-‐Catalyzed Addi3on of Water • Protona3on of C=O makes it more electrophilic Nucleophilic Addi3on of Alcohols: Acetal Forma3on • Alcohols are weak nucleophiles but acid promotes addi3on forming the conjugate acid of C=O • Addi3on yields a hydroxy ether, called a hemiacetal (reversible); further reac3on can occur • Protona3on of the –OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal Uses of Acetals • Acetals can serve as protec3ng groups for aldehydes and ketones • It is convenient to use a diol to form a cyclic acetal (the reac3on goes even more readily) The Reac3ons of Aldehydes and Ketones with Alcohols Acid-‐Catalyzed Hydrolysis of an Acetal The Addi3on of Sulfur Nucleophiles Thioacetals are Desulfurized with Hydrogen and Raney Nickel Addi3on of H–Y to C=O • Reac3on of C=O with H-‐Y, where Y is electronega3ve, gives an addi3on product (“adduct”) • Forma3on is readily reversible Nucleophilic Addi3on of Amines: Imine and Enamine Forma3on • RNH2 adds to R’2C=O to form imines, R’2C=NR (a[er loss of HOH) • R2NH yields enamines, R2N⎯CR=CR2 (a[er loss of HOH) (ene + amine = unsaturated amine) Aldehydes and Ketones Form Imines with Primary Amines Mechanism of Forma3on of Imines • Primary amine adds to C=O • Proton is lost from N and adds to O to yield an amino alcohol (carbinolamine) • Protona3on of OH converts it into water as the leaving group • Result is iminium ion, which loses proton • Acid is required for loss of OH– too much acid blocks RNH2 Forma3on of Imine Deriva3ves Imine Hydrolysis is Irreversible • The amine is protonated in the acidic solution, so it is unable to react with the carbonyl compound. Imine Deriva3ves • Addi3on of amines with an atom containing a lone pair of electrons on the adjacent atom occurs very readily, giving useful, stable imines • For example, hydroxylamine forms oximes and 2,4-‐ dinitrophenylhydrazine readily forms 2,4-‐ dinitrophenylhydrazones – These are usually solids and help in characterizing liquid ketones or aldehydes by mel3ng points Aldehydes and Ketones Form Enamines with Secondary Amines Enamine Forma3on Nucleophilic Addi3on of Hydrazine: The Wolff–Kishner Reac3on • Treatment of an aldehyde or ketone with hydrazine, H2NNH2, and KOH converts the compound to an alkane • Originally carried out at high temperatures but with dimethyl sulfoxide as solvent takes place near room temperature Enamine Hydrolysis is Irreversible • The amine is protonated in the acidic solu3on, so it is unable to react with the carbonyl compound. Reduc3ve Amina3on • The unstable imine formed from ammonia is hydrogenated to an amine. • Imines and enamines are reduced to amines with NaBH3CN. Nucleophilic Addi3on of Phosphorus Ylides: The Wing Reac3on • The sequence converts C=O to C=C • A phosphorus ylide adds to an aldehyde or ketone to yield a dipolar intermediate called a betaine • The intermediate spontaneously decomposes through a four-‐ membered ring to yield alkene and triphenylphosphine oxide, (Ph)3P=O • Forma3on of the ylide is shown below Synthesizing Alkenes (The Wing Reac3on) Mechanism of the Wing Reac3on Uses of the Wing Reac3on • Can be used for monosubs3tuted, disubs3tuted, and trisubs3tuted alkenes but not tetrasubs3tuted alkenes The reac3on yields a pure alkene of known structure • For comparison, addi3on of CH3MgBr to cyclohexanone and dehydra3on with, yields a mixture of two alkenes Choosing Partners for the Wing Reac3on Biological Reduc3ons • The adduct of an aldehyde and OH-‐ can transfer hydride ion to another aldehyde C=O resul3ng in a simultaneous oxida3on and reduc3on (dispropor2ona2on) Direct vs. Conjugate Nucleophilic Addi3on to α,β-‐Unsaturated Aldehydes and Ketones Kine3c and Thermodynamic Control Conjugate Addi3on of Amines • Primary and secondary amines add to α, β-‐unsaturated aldehydes and ketones to yield β-‐amino aldehydes and ketones Conjugate Addi3on of Alkyl Groups: Organocopper Reac3ons • Reac3on of an α,β-‐unsaturated ketone with a lithium diorganocopper reagent • Diorganocopper (Gilman) reagents form by reac3on of 1 equivalent of cuprous iodide and 2 equivalents of organolithium • 1°, 2°, 3° alkyl, aryl and alkenyl groups react but not alkynyl groups Mechanism of Alkyl Conjugate Addi3on: Organocopper Reac3ons • Conjugate nucleophilic addi3on of a diorganocopper anion, R2Cu-‐, to an enone • Transfer of an R group and elimina3on of a neutral organocopper species, RCu Spectroscopy of Aldehydes and Ketones • Infrared Spectroscopy • Aldehydes and ketones show a strong C=O peak 1660 to 1770 cm-‐1 • aldehydes show two characteris3c C–H absorp3ons in the 2720 to 2820 cm-‐1 range. NMR Spectra of Aldehydes • Aldehyde proton signals are near δ 10 in 1H NMR -‐ dis3nc3ve spin–spin coupling with protons on the neighboring carbon, J ≈ 3 Hz 13C NMR of C=O • C=O signal is at δ 190 to δ 215 • No other kinds of carbons absorb in this range Mass Spectrometry – McLafferty Rearrangement • Alipha3c aldehydes and ketones that have hydrogens on their gamma (γ) carbon atoms rearrange as shown Mass Spectroscopy: α-‐Cleavage • Cleavage of the bond between the carbonyl group and the α carbon • Yields a neutral radical and an oxygen-‐ containing ca3on