* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mechanistic notation
Kinetic resolution wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Marcus theory wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
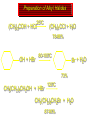
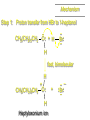
Mechanistic notation The mechanism just described is an example of an SN1 process. SN1 stands for substitution-nucleophilicunimolecular. The molecularity of the rate-determining step defines the molecularity of the overall reaction. Mechanistic notation The molecularity of the rate-determining step defines the molecularity of the overall reaction. H + (CH3)3C O + H Rate-determining step is unimolecular dissociation of alkyloxonium ion. Effect of Alcohol Structure on Reaction Rate slow step is: ROH2+ R+ + H2O The more stable the carbocation, the faster it is formed. Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. Hammond's Postulate If two succeeding states (such as a transition state and an unstable intermediate) are similar in energy, they are similar in structure. Hammond's postulate permits us to infer the structure of something we can't study (transition state) from something we can study (reactive intermediate). carbocation formation carbocation capture R+ proton transfer ROH + ROH2 RX carbocation formation R+ proton transfer ROH + ROH2 Rate is carbocation governed by capture energy of this transition state. Infer structure of this transition state from structure of state of closest energy; in this case the nearest state is the RXcarbocation. 4.13 Reaction of Primary Alcohols with Hydrogen Halides. The SN2 Mechanism Preparation of Alkyl Halides (CH3)3COH + HCl 25°C (CH3)3CCl + H2O 78-88% OH + HBr 80-100°C Br + H2O 73% CH3(CH2)5CH2OH + HBr 120°C CH3(CH2)5CH2Br + H2O 87-90% Preparation of Alkyl Halides Primary carbocations are too high in energy to allow SN1 mechanism. Yet, primary alcohols are converted to alkyl halides. Primary alcohols react by a mechanism called SN2 (substitution-nucleophilic-bimolecular). CH3(CH2)5CH2OH + HBr 120°C CH3(CH2)5CH2Br + H2O 87-90% The SN2 Mechanism Two-step mechanism for conversion of alcohols to alkyl halides: (1) proton transfer to alcohol to form alkyloxonium ion (2) bimolecular displacement of water from alkyloxonium ion by halide Example CH3(CH2)5CH2OH + HBr 120°C CH3(CH2)5CH2Br + H2O Mechanism Step 1: Proton transfer from HBr to 1-heptanol CH3(CH2)5CH2 .. O: + H .. : Br .. H fast, bimolecular H + CH3(CH2)5CH2 O : H Heptyloxonium ion + .. – : Br: .. Mechanism Step 2: Reaction of alkyloxonium ion with bromide ion. H .. – + : Br: + CH3(CH2)5CH2 O : .. H slow, bimolecular H CH3(CH2)5CH2 .. Br .. : 1-Bromoheptane + :O: H + – Br CH2 OH2 CH3(CH2)4 CH2 proton transfer ROH + ROH2 RX 4.14 Other Methods for Converting Alcohols to Alkyl Halides Reagents for ROH to RX Thionyl chloride SOCl2 + ROH RCl + HCl + SO2 Phosphorus tribromide PBr3 + 3ROH 3RBr + H3PO3 Examples CH3CH(CH2)5CH3 SOCl2 K2CO3 CH3CH(CH2)5CH3 Cl OH (81%) (pyridine often used instead of K2CO3) (CH3)2CHCH2OH PBr3 (CH3)2CHCH2Br (55-60%)