* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mechanism of Enzyme Action
Fatty acid metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Genetic code wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Proteolysis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
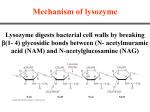
Dr. Ketki K, MBBS, MD Assistant Professor Department of Biochemistry Mechanism of Enzyme Action • Prerequisites for catalysis by enzymes: Lowering of activation energy/ Transition state theory: ? Activation energy: Energy required by the reactant to undergo the reaction is known as activation energy (OR) energy required to convert all molecules of reacting substance from ground state to transition state The reactants when heated, attain the activation energy Lowering of activation energy/ Transition state theory: • Enzyme(Catalyst) lowers the activation energy so that, reaction proceeds at a lower temperature • In other words, enzymes lower the energy barrier of reactants, so that reaction goes faster at body temperature(below40 degree C) A+B AB* C+D E+S ES E+P Reactants Transition state Product Enzyme Stabilizes Transition State Energy change EST S ES EP Reaction direction T = Transition state P Energy decreases (under catalysis) Energy required (no catalysis) ST What’s the difference? Adapted from Alberts et al (2002) Molecular Biology of the Cell (4e) p.166 Mechanism of enzyme action Active Site • Small region on enzyme, at which substrate binds and participates in catalysis Salient features: • 3D structure • Existence is due to tertiary structure of protein resulting in three dimensional native conformation • Cleft / crevices / pockets occupy a small portion of big enzyme • Flexible • Substrate binding site and catalytic site • Substrate binds at the active site non-covalently, • Forces are hydrophobic in nature • The amino acids participate in making & breaking the bonds at the active site are c/a catalytic residues • Amino acids far apart from each other in linear amino acid sequence Amino acids at catalytic site Name of the enzyme Chymotrypsin Trypsin Important amino acids at the catalytic site His (57),Asp(102),Ser(95) Alkaline phosphatase Serine, Histidine, Arginine, Lysine Serine Carbonic anhydrase Cysteine Hexokinase Histidine Different Enzyme Models/ theory • Michaelis and Menten theory • Fischer’s Lock and key model • Koshland’s Induced Fit Model Michaelis and Menten theory/ enzyme substrate complex theory • Example of alkaline Phosphatase: active center of it contains serine residues E- Serine-OH + Glucose-6-P E-Serine-O-P + Glucose E-Serine-O-P E-Serine-OH + Pi Glucose-6-P Glucose + Pi Fischer’s Lock and Key Model P S + + S P E + S ES complex E + P Koshland’s Induced Fit Model P S S P E + S ES complex E + P Active site 1) Defination 2) Salient features 3) Aminoacids at active site/catalytic sitetable 4) Different enzyme models/theory Mechanism of Catalysis • Acid - Base catalysis : In this process the enzyme gives or takes H+ to bring about catalysis. (At physiological pH, histidine is the most important amino acid, the protonated form of which functions as an acid and its corresponding conjugate as a base) Example of acid base catalysis: Action of ribonuclease Histidine residues 12 and 119 at the active site of ribonuclease function as acid & base respectively and donate proton & accept proton respectively. • Substrate strain • Substrate binding to active site induces a strain to the substrate during which the energy level of substrate is raised, leading to a transition state. Eg: Lysozyme • Covalent catalysis • The negatively charged or positively charged groups are present at the active site of the enzyme attack the substrate that results in the covalent binding of the substrate to the enzyme Eg: Serine proteases • Proximity catalysis(proximity effect/entropy effect) • Reactant should come in close proximity to enzyme, for appropriate catalysis to occur • Higher the concentration of substrate molecules, greater will be the rate of reaction. Product substrate orientation theory Enzyme has appropriate three dimensional structure to keep the substrates in a specific orientation, such that reactive groups come to physical apposition. Eg: Glucose + ATP = Glucose-6-P Enzyme Specificity Group Specificity One enzyme can catalyze the same reaction on a group of structurally similar compounds Eg: Hexokinase can catalyze phosphorylation of glucose, galactose & mannose Absolute specificity • Some enzymes are absolutely specific Eg:1)Glucokinase: Phosphorylates only glucose Glucose + ATP ---------------→ Glucose – 6 – phosphate + ADP 2)Galactokinase: Phosphorylates only galactose Galactose + ATP ---------------→ Galactose – 1 – phosphate + ADP 3) Urea is the only substrate for urease Optical specificity Enzymes act only on one isomer L – amino oxidase L-amino acid ------------------→ α – keto acid + NH3 FMN FMNH2 D – amino acid oxidase D–amino acid ------------------→ α - keto acid + NH3 FAD FADH2 Bond specificity • Proteolytic enzymes show bond specificity Eg: Trypsin can hydrolyze peptide bonds formed by carboxyl group of arginine or lysine residues in any protein Attention Check !! A. The active site is (1) the enzyme (2) a section of the enzyme (3) the substrate B. In the induced fit model, the shape of the enzyme when substrate binds (1) stays the same (2) adapts to the shape of the substrate Answers A. The active site is (2) a section of the enzyme B. In the induced fit model, the shape of the enzyme when substrate binds (2) adapts to the shape of the substrate Attention Check !! A. Enzyme trypsin is specific for (1) Arginine and Lysine (2) Phenylalanine and Tryptophan (3) Alanine and Glycine B. The reaction that occurs in substrate strain is (1) Conversion to transition state (2) Chemical reaction Answers A. Enzyme trypsin is specific for (1) Arginine and Lysine B. The reaction that occurs in substrate strain is (1) Conversion to transition state CLINICAL NOTE • Because most vitamins function as coenzymes, the symptoms of vitamin deficiencies reflect the loss of specific enzyme activities that depend on the coenzyme form of the vitamin. • Thus, drugs and toxins that inhibit proteins required for coenzyme synthesis (e.g., vitamin transport proteins or biosynthetic enzymes) can cause the symptoms of a vitamin deficiency. • This type of deficiency is called a functional deficiency, whereas an inadequate intake is called a dietary deficiency. • Most coenzymes are tightly bound to their enzymes and do not dissociate during the course of the reaction. • However, a functional or dietary vitamin deficiency that decreases the level of a coenzyme will result in the presence of the apoenzyme in cells (an enzyme devoid of coenzyme). • Ethanol is an “antivitamin” that decreases the cellular content of almost every coenzyme. For example, ethanol inhibits the absorption of thiamine, and acetaldehyde produced from ethanol oxidation displaces pyridoxal phosphate from its protein-binding sites, thereby accelerating its degradation. CLINICAL NOTE • Many alcoholics develop thiamine deficiency because alcohol inhibits the transport of thiamine through the intestinal mucosal cells. • In the body, thiamine is converted to thiamine pyrophosphate (TPP). TPP acts as a coenzyme in the transamination & decarboxylation reactions of amino acid metabolism and in the utilization of pentose phosphates in the pentose phosphate pathway. • As a result of thiamine deficiency, Dysfunction occurs in the central and peripheral nervous system and other organs. CLINICAL NOTE • In humans, most of ingested ethanol is oxidized to acetaldehyde in the liver by alcohol dehydrogenase (ADH) • ADH is active as a dimer, with an active site containing zinc present in each subunit. The human has at least seven genes that encode isozymes of ADH, each with a slightly different range of specificities for the alcohols it oxidizes. • The acetaldehyde produced from ethanol is highly reactive, toxic, and immunogenic. In patients with chronic alcoholism, acetaldehyde is responsible for much of the liver injury associated with chronic alcoholism. •• "Education "Education is is the the best best friend. friend. An Aneducated educated person person is is respected respected everywhere. everywhere. Education Educationbeats beats the the beauty beauty and andthe the youth." youth." Chanakya Chanakyaquotes quotes (Indian (Indianpolitician, politician, strategist strategistand andwriter, writer,350 350BC-275BC) BC-275BC)