* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Some application of d block metal in biology
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Genetic code wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Western blot wikipedia , lookup
Expanded genetic code wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein adsorption wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
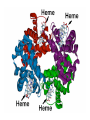
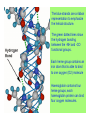
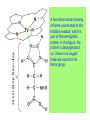
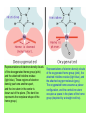
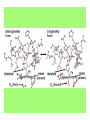
Some application of d block metal in biology By Prof Yeap Guan Yeow Important biological elements At least 25 elements:C, H, N, O Na, K, Mg, Ca, P, S, Cl, Si and Fe V, Cr, Mn, Co, Ni, Cu, Zn, Mo, W, Se, F and I Biomolecules A molecule that naturally occurs in living organisms. Different types: Small molecules: - Lipid, Phospholipid, glycolipid, Sterol Vitamin Hormone, Neurotransmitter Carbohydrate, Sugar Disaccharide Monomers: - Amino acid Nucleotide Phosphate Monosaccharide Polymers: - Peptide, Oligopeptide, Polypeptide, Protein Nucleic acid, I.d. DNA, RNA Oligosaccharide, Polysaccharide Lignin LIPIDS are broadly defined as any fat-soluble (lipophilic), naturally-occurring molecule, such as fats, oils, waxes, cholesterol, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The main biological functions of lipids include energy storage, acting as structural components of cell membranes, and participating as important signaling molecules. Amino Acid In chemistry, an amino acid is a molecule containing both amine and carboxyl functional groups. In biochemistry, this term refers to alpha-amino acids with the general formula H2NCHRCOOH, where R is an organic substituent. Example: Phenylalanine Peptides (from the Greek π_π_____, "small digestibles") (i) Short polymers formed from the linking, in a defined order, of _-amino acids. (ii) The link between one amino acid residue and the next is known as an amide bond or a peptide bond. Less abundant species Na, K and Cl for osmotic control and nerve action Mg2+ in chlorophyll Ca for bone formation For transition metal complexes: Fe - Ferritin, Transferrin, Haemoglobin Co - Vitamin B12 Cu - Haemocyanin Zn Ferritin - A water-soluble metalloprotein in which the Fe is stored in the liver, bone marrow and spleen. - Contains up to 4500 high-spin Fe3+ in the form of microcrystalline oxohydroxophosphate of composition (FeO.OH)8(FeO.H2PO4) - Can release iron if the blood has a low Fe concentration, and it can help to store excess Fe if the blood and tissues have a high Fe concentration. - Functions as a "buffer" against Fe deficiency and, to a lesser extent, against iron overload. Protein shell in ferritin Ferritin (a) Ferritin has the shape of a hollow sphere. Inside the sphere, Fe is stored in the Fe(III) oxidation state. It is incorporated in the mineral ferrihydrite, [FeO(OH)]8[FeO(H2PO4)], which is attached to the inner wall of the sphere. (b) To release Fe when the body needs it, the Fe must be changed from the Fe(III) to the Fe(II) oxidation state. (c) In the Fe(II) state, iron breaks away from the lattice as the Fe2+ ion. The positive charge of the Fe2+ ion attracts the electronegative O atoms of H2O, and a H2O "cage" forms around the ion, with six H2O molecules surrounding the ion . (d) Fe becomes soluble as a hydrated Fe2+ ion , Fe(H2O)62+, and it can be released from the ferritin protein via the channels in the spherical shell. Transferrin A glycoproteins (ie. Compounds of proteins and carbohydrates) which binds Fe very tightly but reversibly. The affinity of transferrin for Fe(III) is extremely high (1023 M-1 at pH 7.4) but decreases progressively with decreasing pH below neutrality. When not bound to iron, it is known as "apo-transferrin" Include serum transferrin, lactoferrin (in milk) and ovotransferrin (present in egg white) Binding site in transferrin Siderophores (i) Organisms with O-donor polydentate ligand called siderophores scavenge for Fe. (ii) Examples of siderophores are anions derived from:- enterobactin - desferrichrome - desferrioxamine Enterobactin (also known as Enterochelin) is a high affinity siderophore that acquires iron for microbial systems. It is primarily found in gram-negative bacteria, such as Escherichia coli and Salmonella typhimurium. It is the strongest siderophore known, binding to the ferric ion (Fe3+) with the affinity (K = 1052 M-1). Haemoglobin & Myoglobin - Both are haem-iron myoglobin Haemoglobin - Protein that transports oxygen (O2 ) in human blood from the lungs to the tissues of the body - Molecular weight of ~64500 - Is a globular protein (i.e., folded into a compact, nearly spherical shape) and consists of four subunits - A tetramer Upon the reaction of O2 with haemoglobin: - Coordination is accompained by electron transfer - High-spin Fe(II) was oxidized to low-spin Fe(III) and reducing O2 to [O2]- The blue strands are a ribbon representation to emphasize the helical structure. The green dotted lines show the hydrogen bonding between the -NH and -CO functional groups. Each heme group contains an iron atom that is able to bind to one oxygen (O2) molecule Haemoglobin contains four heme groups, each haemoglobin protein can bind four oxygen molecules. Porphyrin Heterocyclic macromolecules that can serve as a tetradentate ligand. a three-dimensional molecular model of heme coordinated to the histidine residue (a monodentate ligand) of the hemoglobin protein A two-dimensional drawing of heme coordinated to the histidine residue, which is part of the hemoglobin protein. In this figure, the protein is deoxygenated; i.e., there is no oxygen molecule bound to the heme group. Conformational Changes Upon Binding of Oxygen In deoxygenated state: The heme group is nonplanar when it is in its deoxygenated state (not bound to an oxygen molecule); the iron atom is pulled out of the plane of the porphyrin toward the histidine residue to which it is attached. This nonplanar configuration is characteristic of the deoxygenated heme group and is commonly referred to as a "domed" shape. The valence electrons in the atoms surrounding iron in the heme group and the valence electrons in the histidine residue form "clouds" of electron density. (Electron density refers to the probability of finding an electron in a region of space.) Because electrons repel one another, the regions occupied by the valence electrons in the heme group and in the histidine residue are pushed apart. Hence, the porphyrin adopts the domed (nonplanar) configuration in which the Fe is out of the plane of the porphyrin ring. In oxygenated state When in the heme group is in its oxygenated state (i.e., the Fe atom is bound to an oxygen molecule), the porphyrin ring adopts a planar configuration in which the Fe lies in the plane of the porphyrin ring. Representations of electron-density clouds of the deoxygenated heme group (pink) and the attached histidine residue (light blue). These regions of electron density push one another apart, and the iron atom in the center is drawn out of the plane. (The bent line represents the nonplanar shape of the heme group.) Representations of electron-density clouds of the oxygenated heme group (pink), the attached histidine residue (light blue), and the attached oxygen molecule (gray). The oxygenated heme assumes a planar configuration, and the central iron atom occupies a space in the plane of the heme group (depicted by a straight red line). Myoglobin - Molecular weight of ~17000 - A monomer with a protein chain consisting of 1 53 amino acid residues