* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 27 timed ppt
Survey
Document related concepts
Transcript
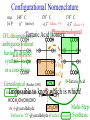
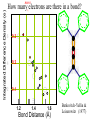
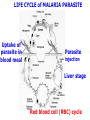
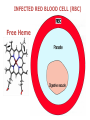
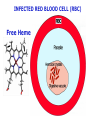
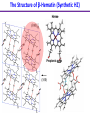
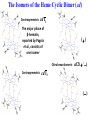
Chemistry 125: Lecture 27 November 5, 2010 Prof. Leiserowitz on Determining Absolute Configuration and the “Stereochemistry of Malaria” Emil Fischer, seeing that it was impossible to determine “absolute” configuration by the methods available, decided to define relative configuration by chemical transformations beginning with D-glyceraldehyde. 60 years later Bijvoet used a special x-ray methods to show that Fischer had guessed right about glyceraldehyde. Professor Leiserowitz explains to the class first how simple x-ray work, together with a knowledge about how “tailor made additives” influence crystal growth, can be used in a simpler way to determine absolute configuration of amino acids, a task that experts thought impossible. He then shows how the same concepts apply in the study of hemozoin, the crystals that malaria parasites use to keep from poisoning themselves with heme. Stopping hemozoin crystallization might help control malaria. The stereochemistry of hemozoin is instructive. For copyright notice see final page of this file Chemistry 125: Lecture 27 November 5, 2010 Communicating Molecular Structure in Diagrams and Words Correct configuration is vital in drug molecules, like eribulin. It is important that chemists agree on notation and nomenclature in order to communicate molecular constitution and configuration. Clear notation also aids clarity of thought. The conventional 1891 Fischer projection, which has been indispensable in understanding sugar configurations for over a century, was invented in order to count stereoisomers. Ambiguity in diagrams or words has led to multibillion dollar patent disputes involving popular pharmaceuticals. International agreements provide descriptive, unambiguous, unique, systematic “IUPAC” names that are reasonably convenient for most organic molecules of modest molecular weight. Also in 1891 Fischer devised the D,L “genealogical” scheme to describe relative configurations, but it can be cumbersome or ambiguous. Preliminary For copyright notice see final page of this file Configurational Nomenclature m.p. [a]D 140°C 0° (meso) 170°C +13° (dextro +) 170°C -13° (laevo -) Phenomenological Tartaric Acid Isomers D/L designation is ambiguous without having a detailed ? synthesis recipe Why or a convention. not? D-Tartaric Acid Genealogical (Fischer, 1891) Impossible to know ? which is which! Relative (by synthesis) to HOCH2CH(OH)CHO d-(+)-glyceraldehyde Defined as “D”-glyceraldehyde (Fischer’s Guess) Multi-Step Synthesis Impossible to know which is which? Absolute Configuration J. M. Bijvoet van't Hoff Laboratory, Univ. Utrecht (1949-51) Na Rb d-(L)-Tartrate X-ray anomalous dispersion 60 year old Fischer Guess for of our “The question of nomenclature is beyond the scope investigation... The problem of nomenclature (L)-Tartrate now concerns given configurations, and requires a notation which denotes these configurations in an unambiguous and if possible selfexplanatory way.” (Bijvoet, 1951) Lewis Bookkeeping Density (e) Differenceelectrons Integrated more How many electrons are there in a bond? ^ 6 0.3 4 0.2 2 0.1 1.2 1.4 1.6 Bond Distance (Å) Berkovitch-Yellin & Leiserowitz (1977) Professor Leiserowitz has subsequently applied his skills at solving challenging problems in x-ray diffraction, crystal packing, and crystal growth to address the question of determining absolute chirality and more recently the possibility that preventing crystal nucleation could make malaria parasites poison themselves with the dissolved heme by-product they generate by living off their host’s hemoglobin. He gives the class a brief synopsis of some aspects of this recent work. Effect of Capping Molecules and TailorMade Additives on Crystal Growth Nucleation of Crystalline Hemozoin in the Malaria-Infected Red Blood Cell Leslie Leiserowitz, Dept of Materials & Interfaces, The Weizmann Institute of Science Geographic Distribution of Malaria in the World (1989) Reproduced from the book, Malaria: Obstacles and Opportunities MALARIA REEMERGING INFECTIOUS DISEASE 300106 infections per year, 1-2106 deaths, mostly children and pregnant women •Poverty: Inadequate housing and water control. Lack of bed nets (impregnated with slowly released mosquito repellant) •The Anopheles mosquito has developed resistance to insecticides Malaria parasites have developed resistance to commonly-used synthetic quinoline drugs LIFE CYCLE of MALARIA PARASITE Uptake of parasite in blood meal Parasite injection Liver stage Red blood cell (RBC) cycle INFECTED RED BLOOD CELL (RBC) Free Heme INFECTED RED BLOOD CELL (RBC) Free Heme The Structure of β-Hematin (Synthetic HZ) Heme C D Propionic acids Formation of the different Cyclic Heme Dimer Isomers (cd). Schematic Representation. methyl vinyl vinyl C D axis of pseudo symmetry methyl Note: The dimers form as a sort of slipped sandwich held together by one of two propionic acid groups (left or right) of the lower heme (both of which project toward the viewer) binding to the Fe atom in the center of the upper heme. At the same time one of the two acids (right or left) of the upper heme (both of which project away from the viewer) binds to Fe of the lower heme. So in addition to the substituents of each heme being arranged either clockwise or counterclockwise, the center of the dimer could have the upper heme offset to the right or to the left of the lower heme. The problem is to figure out how many isomers are possible. This is a bit more complex that van’t Hoff’s tartaric acid problem in frame 5 of Lecture 26. See if you can figure out how many stereoisomers are possible. Do you agree with Prof. Leiserowitz? The Isomers of the Heme Cyclic Dimer (cd) Centrosymmetric cd 11 The major phase of β-hematin, reported by Pagola et al., consists of one isomer ( ) Chiral-enantiomeric Centrosymmetric cd 2( / ) cd 12 ( ) β-hematin crystal structure study β-hematin crystals precipitated quickly within 1-2 days vs. several days or weeks by Pagola et al. Powder X-ray data collected at Spring-8, Japan by T. Straasø, University of Copenhagen. Powder sample cooled down to 100 K to reduce thermal motion. Better characterization of possible molecular disorder β-hematin: X-ray powder data peaks not explained by the Pagola dimer structure β-hematin: minor phase. Five peaks Major phase Minor phase c a b a 12.08 14.48 7.99 90.83 96.9 97.6 12.39 15.10 7.61 99.50 96.8, 93.5 According to XRPD analysis minor phase 13% of total amount XRPD of biogenic hemozoin crystals shows only one crystalline phase. May be related to the state of heme in hemoglobin (which is 4 hemes plus the protein that the parasite eats). cd2(+) Structure of hemoglobin shows O2 bound to heme on one side only. In which case primarily only the enantiomeric chiral heme dimer cd2(+) would be formed End of Lecture 27 Nov. 5, 2010 Copyright © J. M. McBride 2009,2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0