* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1-Structure of Heme
Protein design wikipedia , lookup
Cooperative binding wikipedia , lookup
Implicit solvation wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Homology modeling wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein domain wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein purification wikipedia , lookup
Western blot wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein folding wikipedia , lookup
Alpha helix wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
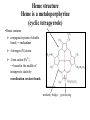
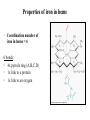
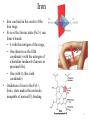
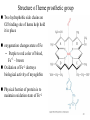
Structure of Heme Dr. Shumaila Asim Lecture # 1 1 Hemoproteins • Hemoproteins are a group of specialized proteins that contain heme as a tightly bound prosthetic group. • Heme is a complex of protoporphyrin IX and ferrous iron (Fe2+) . • The iron is held in the center of the heme molecule by bonds to the four nitrogens of the porphyrin ring. 2 Heme is a complex of protoporphyrin IX and ferrous iron (Fe2+) Hemoproteins • Hemoglobin (Hb) • Myoglobin (Mb) • Cytochromes • Catalases (decomposition of 2 H2O2 to 2 H2O and O2) • Peroxidases Globular proteins • Amino acid chains fold into shapes that resemble spheres are called globular proteins • This type of folding increases solubility of proteins in water – Polar groups on the protein’s surface – Hydrophobic groups in the interior • Fibrous proteins are mainly insoluble structural proteins 5 Four Levels of Protein Structure • Primary, 1o – the amino acid sequence • Secondary, 2o – 2-D arrangement of backbone atoms in space • Tertiary, 3o – 3-D arrangement of all the atoms in space • Quaternary, 4o – 3-D arrangement of subunit chains 6 Globin of hemoglobin is a globular protein with a quaternary structure 7 Globular proteins • Hemoglobin: oxygen transport function • Myoglobin: oxygen storage/supply function in heart and muscle • α1, α2, β-globulins: various functions • γ-globulins (immunoglobulins): immune function • Enzymes: catalysis of biochemical reactions 8 Hemoglobin A major globular protein in humans Composed of four polypeptide chains: Two α and two β chains Contains two dimers of αβ subunits Held together by non-covalent interactions Each chain is a subunit with a heme group in the center that carries oxygen A Hb molecule contains 4 heme groups and carries 4 molecules of O2 9 10 Heme is the prosthetic group of hemoglobin, myoglobin, & cytochromes. Heme is an asymmetric molecule. E.g., note the positions of methyl side chains around the ring system. 11 Heme structure Heme is a metaloporphyrine (cyclic tetrapyrrole) •Heme contains: conjugated system of double bonds → red colour 4 nitrogen (N) atoms 1 iron cation (Fe2+) → bound in the middle of tetrapyrrole skelet by coordination covalent bonds methenly bridge pyrrole ring Properties of iron in heme • Coordination number of iron in heme = 6 6 bonds: • 4x pyrrole ring (A,B,C,D) • 1x link to a protein • 1x link to an oxygen Properties of iron in heme Myoglobin (Mb) • is a single-chain globular protein of 153 AA, containing 1 heme group • transports O2 in skeletal and heart muscle • is found in cytosol within cells • is a marker of myocard damage Iron • Iron can bind in the center of the four rings. • Fe is in the ferrous state (Fe2+) can form 6 bonds: – 4 with the nitrogen of the rings, – One (known as the fifth coordinate) with the nitrogen of a histidine imidazole (known as proximal His). – One with O2 (the sixth coordinate) • Oxidation of iron to the Fe3+, ferric, state makes the molecule incapable of normal O2 binding 17 Structure-function relationship • The planar heme group fits into a hydrophobic pocket of the protein and the myoglobin-heme interaction is stabilized by hydrophobic attractions. • The heme group stabilizes the tertiary structure of myoglobin. • The hydrophobic interior of myoglobin (or hemoglobin) prevents the oxidation of iron, and so when O2 is released, the iron remains in the Fe(II) state and can bind another O2. 18 The distal histidine acts as a gate that opens and closes as O2 enters the hydrophobic pocket to bind to the heme Structure of heme prosthetic group Two hydrophobic side chains on O2 binding site of heme help hold it in place oxygenation changes state of Fe – Purple to red color of blood, Fe+3 – brown Oxidation of Fe+2 destroys biological activity of myoglobin Physical barrier of protein is to maintain oxidation state of Fe+2 20