* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Plasma Proteins - neutralposture
Gel electrophoresis wikipedia , lookup
Degradomics wikipedia , lookup
Protein domain wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein folding wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein purification wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein–protein interaction wikipedia , lookup
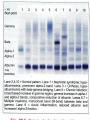
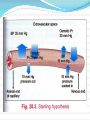
Plasma Proteins Prof Dr Kwan Teck Kim Department of Biochemistry Faculty of Medicine Mahsa University College Learning outcomes On completion of this topic, the student will be able to: Explain the basis of classification of plasma proteins Name the major types of plasma proteins Discuss the major roles of plasma proteins Contents Plasma proteins Electrophoresis Albumin, functions, clinical significance Hypo-albuminemia Globulins, alpha, beta, gamma Transport proteins in blood Acute phase proteins in blood Ceruloplasmin Alpha-1 anti-trypsin Clotting factors Protein molecules in blood Electrophoresis Employed for separation of serum proteins Movement of charged particles through an electrolyte when subjected to an electric field Positively charged particles (cations) move to cathode Negatively charged particles (anions) move to anode At pH 8.6 (barbitone buffer), serum proteins will have net negative charge and migrate towards anode. Electrophoresis (contd) • In agar gel electrophoresis, normal serum is separated into 5 bands: • Albumin : 55-65% • Alpha-1 globulin : 2-4% • Alpha-2 globulin : 6-12% • Beta-globulin : 8-12% • Gamma-globulin : 12-22% Albumin has the maximum and gamma globulin has the minimum mobility in the electrical field. γ- globulins contain the antibodies (immunoglobulins), Most of α-1 fraction is made up of α-1-antitrypsin; α-2 band is mainly made up by α-2-macroglobulin. β-fraction contains low density lipoproteins. Anode Cathode Electrophoretogram Functions of Albumin 1. i. Colloid Osmotic Pressure of Plasma Proteins cannot easily escape out of blood vessels, & therefore, proteins exert the ‘effective osmotic pressure’. It is about 25 mm Hg, & 80% of it is contributed by albumin. ii. According to Starling’s hypothesis, at the capillary end the BP or hydrostatic pressure expels water out, & effective osmotic pressure (EOP) takes water into the vascular compartment. iii. At the arterial end of capillary, BP is 35 mm Hg & EOP 25 mm; thus water is expelled by a pressure of 10mm Hg. iv. At the venous end of capillary, EOP is 25 mm and BP is 15 mm, water is imbibed with a pressure of 10 mm. Albumin (contd 1) v. If protein concn in serum is reduced, the EOP is correspondingly decreased. Then return of water into blood vessels is diminished, leading to accumulation of water in tissues (edema). 2. Transport Function i. Bilirubin & non-esterified fatty acids are specifically transported by albumin. ii. Drugs (supha, aspirin, salicylates, dicoumarol, pheytoin) iii. Hormones (Steroid hormones, thyroxine) iv. Metals (Ca, Cu) and heavy metals are nonspecifically carried by albumin. Albumin (contd 2) 3. Nutritional Function All tissue cells can take up albumin by pinocytosis. It is then broken down to amino acid level. So, albumin may be considered as the transport form of essential amino acids from liver to other tissues. Plasma Proteins Total blood volume is ~ 4.5 – 5 litres Defibrinated plasma is called serum Total protein of normal plasma, 6-8 g/100 ml Consists of 3.5-5.0 g/dl albumin; 2.5-3.5 g/dl globulins and 200-400 mg/dl fibrinogen Plasma proteins (contd) The albumin : globulin ratio, 1.2 : 1 to 1.5 : 1 Hypo-albuminemia is seen in cirrhosis, chronic liver failure, malnutrition, malabsorption diseases, nephrotic syndrome, burns All (except immunoglobulins) are synthesized in liver; mostly glycoproteins Abnormal Patterns in Clinical Diseases 1. Chronic infections: The γ globulins are increased, but increase is smooth and wide-based 2. Multiple myeloma: In para-proteinemias, a sharp spike termed M-Band. 3. Nephrotic syndrome: All proteins except very big molecules are lost through urine. Alpha-2 fraction (containing macroglobulin) is very prominent Functions of Plasma Proteins 1. Albumin: chief stabiliser of blood volume and helps in transport of drugs, bilirubin, free fatty acids, calcium, hormones & metals serves as a source amino acids for tissue protein synthesis helps in maintaining colloidal osmotic pressure; buffering capacity (see earlier slides no. 11-13) 2. Gobulins: α 1 –globulin (retinol binding protein) involved in transport of vitamin A α 2 –globulin (ceruloplasmin) involved in transport of copper β-globulin (transferrin) an iron transport protein γ-globulins (antibodies) confer immunity against infectious diseases Fibrinogen: Participates in blood coagulation Acute phase proteins 1. C-Reactive Protein (CRP) β-globulin, 115-140 kD, stimulates complement activity & macrophage phagocytosis has a positive correlation in predicting the risk of coronary artery diseases 2. Ceruloplasmin α2-globulin, 160 kD; 6-8 copper atoms per molecule; also called Ferroxidase which helps in incorporation of Fe into transferrin. An important anti-oxidant in plasma 2. Ceruloplasmin (contd) 90% Cu in plasma is bound to ceruloplasmin 10% Cu bound with albumin loosely Lower level of ceruloplasmin in Wilson’s disease, malnutrition, nephrosis, cirrhosis Increased level in active hepatitis, biliary cirrhosis, hemochromatosis, obstructive biliary disease 3. Alpha-1 Anti-trypsin (AAT) • Also called Alpha-anti-proteinase or protease inhibitor • It inhibits all serine proteases (proteolytic enzymes having a serine at their active centre), such as plasmin, thrombin, trypsin, chymotrypsin, elastase, cathepsin. • Serine protease inhibitors (Serpins) • AAT is synthesized in liver. A glycoprotein, 50 kD. • Normal serum level 75-200 mg/dl • AAT deficiency causes Emphysema and Nephrotic syndrome. 3. Alpha-1 Anti-trypsin (AAT) [contd] Emphysema: AAT deficiency 1 in 1000 Bacterial infections in lung attract macrophages which release elastase. In AAT deficiency, unopposed action of elastase causes damage to lung tissue, leading to emphysema. About 5% of emphysema cases are due to AAT deficiency Nephrotic syndrome: • AAT molecules are lost in urine • Hence, AAT deficiency is produced 4. Alpha-2-Macroglobulin (AMG) AMG, tetrameric protein, 725 kD Major component of α 2 –globulins Synthesized by hepatocytes and macrophages Inactivates all proteases Important in vivo anti-coagulant Carrier of many growth factors such as platelet derived growth factor (PDGF). Normal serum level is 130-300 mg/dl Its concentration is markedly increased (up to 2-3 g/dl) in Nephrotic syndrome Clotting Factors 1. Prothrombin Single chain zymogen, 69 kD Plasma concentration 10-15 mg/dl Prothrombin → thrombin by Factor Xa, by removal of N-terminal fragment 2. Thrombin Serine protease, 34 kD The Ca2+ binding of prothrombin is essential for anchoring prothrombin on surface of platelets. When terminal fragment is cleaved off, Ca2+ binding sites are removed. So, thrombin is released from platelet surface. Clotting Factors (contd) 3. Fibrinogen An acute phase protein Synthesized by liver, 340 kD Normal fibrinogen level in blood, 200-400 mg/dl Fibrinogen → fibrin by cleaving Arg-Gly peptide bonds of fibrinogen Fibrin monomers formed are insoluble They align themselves lengthwise, aggregate and precipitate to form the clot References • Devlin T.M. [Ed.] (2006), Textbook of Biochemistry with Clinical Correlations, 6th Ed., Wiley-Liss, Inc, N.Y. Vasudeven, D.M., Sreekumari, S. & Vaidyanathan, K. (2011). Textbook of Biochemistry for Medical Students , 6th Ed., Jaypee Brothrs Medical Publishers Ltd, New Delhi. Murray, R.K. et al. (2009) Harper’s Illustrated Biochemistry, 28th Ed., Mc Graw Hill Medical, N.Y.