* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Types of Radiation
Electron configuration wikipedia , lookup
Technetium-99m wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
X-ray fluorescence wikipedia , lookup
History of chemistry wikipedia , lookup
Nuclear fission product wikipedia , lookup
Livermorium wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical element wikipedia , lookup
Elementary particle wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear fission wikipedia , lookup
History of molecular theory wikipedia , lookup
Extended periodic table wikipedia , lookup
Radioactive decay wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Valley of stability wikipedia , lookup
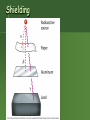
Unstable Nuclei & Radioactive Decay Radioactivity Nucleus of an element spontaneously emits subatomic particles & electromagnetic waves. Nucleus changes into a different element when it does this. Original nucleus is called “unstable.” Process is called “decay” or “transmutation.” Rutherford & Radioactivity 1898 – Rutherford began experiments with radioactivity. 1899 – discovered alpha and beta “rays” from uranium. Types of Radiation Radioactivity – types Characteristics of Radiation See Table O Name Alpha particle Beta particle Gamma radiation Positron emission Symbol or 42He or24 or 0e or 0 or -1 -1 or 00 0 +1 or 0e or + +1 Mass (amu) 4 Relative Charge +2 0 -1 0 0 0 +1 2 Neutrons & 2 Protons. Charge = +2 Mass = 4 Beta Particle – fast moving electron. Radioactive atom: Change occurs in nucleus. Shielding Can we predict exactly when an atom will decay? NO! For large #’s of atoms, we CAN predict how many will decay on average in a given amount of time. Which elements are radioactive? All elements past Bismuth in the periodic table. If the atomic number is 83, it’s radioactive! Other elements may have radioactive isotopes. Stability depends on neutron/proton ratio. applet What’s Going on in the Nucleus? Electrostatic repulsions between protons. Want to fly apart. But protons & neutrons all attracted to each other by nuclear strong force. So having neutrons helps hold a nucleus together. Strong Force Works best if the nucleus isn’t too large. As the nucleus gets larger, need to have more neutrons to help counteract the electrostatic repulsion between the protons. Eventually, the nucleus is too large to be stable. Balancing Act Balance exists between electrostatic repulsive force & nuclear strong force. Certain #’s of protons & neutrons make a stable nucleus. Other #’s of protons & neutrons are unstable. So the atom decays. Beyond Element 83 No amount of neutrons can hold a nucleus together once it has more than 83 protons. Elements 84 & above are radioactive. Stability and the n/p ratio For atoms below atomic number = 20, best neutron/proton ratio 1. As atomic number , atoms need more neutrons to be stable. So n/p ratio for stable atoms increases to 1.5 for big atoms. Stability Line Type of radiation emitted depends on position relative to stable nuclei. Blue: too many neutrons. Yellow: not enough neutrons for the protons. Red/Orange: too many protons and neutrons Natural Radioactivity – Unstable Nuclei Emit Radiation Spontaneous nuclear change to attain good n/p ratio (high stability, low energy state). Form a new kind of atom. Each isotope or nuclide decays in a certain manner to get a better n/p ratio. The decay mode is named for the particle emitted. See Table N. Nuclear Equations Describe the decay process. reactant or starting side (left) product or ending side (right). separates two sides Nuclear vs. Chemical How is a nuclear change different from a chemical change? NUCLEAR Involve a change in an atom’s nucleus. Radioactive atoms spontaneously emit radiation and change into other kinds of atoms. Nuclear reactions involve 1,000,000 X more energy than ordinary chemical rxns. CHEMICAL Involve changes in the outermost electrons. 1 or more substances changed into new substances. Atoms are rearranged, but their identities do not change. Mass Energy In nuclear reactions, mass is converted into energy. – Mass Defect is the difference between the mass of a nucleus and the sum of the masses of its constituent particles. E = 2 mc Binding Energy Energy released when a nucleus is formed from its constituent particles. It is a measure of the stability of an atom formed: – The higher the binding energy the more stable the nucleus. – The lightest and heaviest elements are the most unstable (low BE) – Intermediate elements are the most stable (highest BE). Binding Energy Fe-56 Ni-62