* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Principles of BIOCHEMISTRY
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Mitochondrion wikipedia , lookup
Signal transduction wikipedia , lookup
Microbial metabolism wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Proteolysis wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
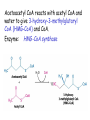
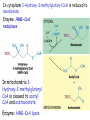
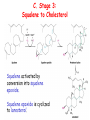
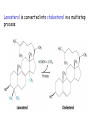
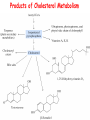
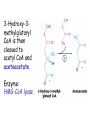
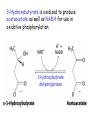
LIPID METABOLISM: CHOLESTEROL METABOLISM Functions of Cholesterol • a precursor of steroid hormones (progesterone, testosterone, estradiol, cortisol, etc.) • a precursor of bile acids • a precursor of vitamin D • important component of many mammalian membranes (modulates the fluidity) Sources of Cholesterol • from the diet • can be synthesized de novo (about 800 mg of cholesterol per day) - in the liver (major site) - in the intestine • Liver-derived and dietary cholesterol are both delivered to body cells by lipoproteins Synthesis of Cholesterol Three stages of cholesterol biosynthesis 1. Synthesis of isopentenyl pyrophosphate, that is the key building block of cholesterol, from acetyl CoA 2. Condensation of six molecules of isopentenyl pyrophosphate to form squalene 3. Squalene cyclizes and the tetracyclic product is converted into cholesterol Acetyl CoA (C2) Isopentenyl pyrophosphate (C5) Squalene (C30) Cholesterol (C27) A. Stage 1: Acetyl CoA to Isopentenyl Pyrophosphate • All carbons of cholesterol come from cytosolic acetyl CoA (transported from mitochondria via citrate transport system) • Sequential condensation of three molecules of acetyl CoA Two molecules of acetyl CoA condense to form acetoacetyl CoA. Enzyme – thiolase. Acetoacetyl CoA reacts with acetyl CoA and water to give 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) and CoA. Enzyme: HMG-CoA synthase In cytoplasm 3-Hydroxy-3-methylglutaryl CoA is reduced to mevalonate. Enzyme: HMG-CoA reductase In mitochondria 3Hydroxy-3-methylglutaryl CoA is cleaved to acetyl CoA and acetoacetate. Enzyme: HMG-CoA lyase. HMG-CoA reductase • HMG-CoA reductase is an integral membrane protein in the endoplasmic reticulum • Primary site for regulating cholesterol synthesis • Cholesterol-lowering statin drugs (e.g. Lovastatin) inhibit HMG-CoA reductase Lovastatin resembles mevalonate Mevalonate is converted into 3-isopentenyl pyrophosphate in three consecutive reactions requiring ATP and decarboxylation. Isopentenyl pyrophosphate is a key building block for cholesterol and many other important biomolecules. B.Stage 2: Isopentenyl Pyrophosphate to Squalene Isopentenyl pyrophosphate is isomerized to dimethylallyl pyrophosphate. C5 units isopentenyl pyrophosphate react with C5 units dimethylallyl pyrophosphate to yield C10 compound geranyl pyrophosphate C10 compound geranyl pyrophosphate reacts with C5 units isopentenyl pyrophosphate and C15 compound is formed - farnesyl pyrophosphate. Reductive tail-to-tail condensation of two molecules of farnesyl pyrophosphate results in the formation of C30 compound squalene C. Stage 3: Squalene to Cholesterol Squalene activated by conversion into squalene epoxide. Squalene epoxide is cyclized to lanosterol. Lanosterol is converted into cholesterol in a multistep process. THE REGULATION OF CHOLESTEROL BIOSYNTHESIS Regulatory enzyme - 3-hydroxy-3-methylglutaryl CoA reductase. Tetrameric enzyme. NADPH coenzyme HMG CoA reductase is controlled in multiple ways: 1. The rate of synthesis of reductase mRNA is controlled by the sterol regulatory element binding protein (SREBP). When cholesterol levels fall this protein migrates to the nucleus and enhance transcription. 2. The rate of translation of reductase mRNA is inhibited by cholesterol 3. The degradation of the reductase is controlled. The increase of cholesterol concentration makes the enzyme more susceptible to proteolysis. 4. Phosphorylation decreases the activity of the reductase. Enzyme is switched off by an AMP-activated protein kinase. Thus, cholesterol synthesis ceases when the ATP level is low. Products of Cholesterol Metabolism ATHEROSCLEROSIS The desirable level of cholesterol in blood plasma: < 200 mg/dl (< 5 mmol/l) For a healthy person, the LDL/HDL ratio is 3.5 KETONE BODIES The entry of acetyl CoA into the citric acid cycle depends on the availability of oxaloacetate. The concentration of oxaloacetate is lowered if carbohydrate is unavailable (starvation) or improperly utilized (diabetes). Oxaloacetate is normally formed from pyruvate by pyruvate carboxylase (anaplerotic reaction). Fats burn in the flame of carbohydrates. In fasting or diabetes the gluconeogenesis is activated and oxaloacetate is consumed in this pathway. Fatty acids are oxidized producing excess of acetyl CoA which is converted to ketone bodies: b-Hydroxybutyrate Acetoacetate Acetone Ketone bodies are synthesized in liver mitochondria and exported to different organs. Ketone bodies are fuel molecules (can fuel brain and other cells during starvation) A. Synthesis of ketone bodies Two molecules of acetyl CoA condense to form acetoacetyl CoA. Enzyme – thiolase. Acetoacetyl CoA reacts with acetyl CoA and water to give 3hydroxy-3methylglutaryl CoA (HMGCoA) and CoA. Enzyme: HMG-CoA synthase 3-Hydroxy-3methylglutaryl CoA is then cleaved to acetyl CoA and acetoacetate. Enzyme: HMG-CoA lyase. 3-Hydroxybutyrate is formed by the reduction of acetoacetate by 3-hydroxybutyrate dehydrogenase. Acetoacetate also undergoes a slow, spontaneous decarboxylation to acetone. The odor of acetone may be detected in the breath of a person who has a high level of acetoacetate in the blood. B. Ketone bodies are a major fuel in some tissues Ketone bodies diffuse from the liver mitochondria into the blood and are transported to peripheral tissues. Ketone bodies are important molecules in energy metabolism. Heart muscle and the renal cortex use acetoacetate in preference to glucose in physiological conditions. The brain adapts to the utilization of acetoacetate during starvation and diabetes. 3-Hydroxybutyrate is oxidized to produce acetoacetate as well as NADH for use in oxidative phosphorylation. 3-hydroxybutyrate dehydrogenase Acetoacetate is activated by the transfer of CoA from succinyl CoA in a reaction catalyzed by a specific CoA transferase. Acetoacetyl CoA is cleaved by thiolase to yield two molecules of acetyl CoA (enter the citric acid cycle). CoA transferase is present in all tissues except liver. Ketone bodies are a watersoluble, transportable form of acetyl units KETOSIS The absence of insulin in diabetes mellitus liver cannot absorb glucose inhibition of glycolysis activation of gluconeogenesis deficit of oxaloacetate activation of fatty acid mobilization by adipose tissue large amounts of acetyl CoA which can not be utilized in Krebs cycle large amounts of ketone bodies (moderately strong acids) severe acidosis (ketosis) Impairment of the tissue function, most importantly in the central nervous system