* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
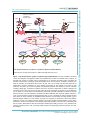
Download Targeting of the immune system in systemic lupus erythematosus
Lymphopoiesis wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Molecular mimicry wikipedia , lookup
Autoimmunity wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Systemic lupus erythematosus wikipedia , lookup
Sjögren syndrome wikipedia , lookup
expert reviews in molecular medicine Targeting of the immune system in systemic lupus erythematosus Meera Ramanujam and Anne Davidson* Systemic lupus erythematosus (SLE) is a complex immune disorder in which loss of tolerance to nucleic acid antigens and other crossreactive antigens is associated with the development of pathogenic autoantibodies that damage target organs, including the skin, joints, brain and kidney. New drugs based on modulation of the immune system are currently being developed for the treatment of SLE. Many of these new therapies do not globally suppress the immune system but target specific activation pathways relevant to SLE pathogenesis. Immune modulation in SLE is complicated by differences in the immune defects between patients and at different disease stages. Since both deficiency and hyperactivity of the immune system can give rise to SLE, the ultimate goal for SLE therapy is to restore homeostasis without affecting protective immune responses to pathogens. Here we review recent immunological advances that have enhanced our understanding of SLE pathogenesis and discuss how they may lead to the development of new treatment regimens. Systemic lupus erythematosus (SLE) is a disease characterised by loss of B-cell tolerance to autoantigens, particularly nucleic acids and their binding proteins. Although autoantibodies to nuclear antigens are elicited in healthy individuals during protective immune responses (Ref. 1) and are present in the serum of up to 20% of the population, they are not sufficient to cause autoimmune disease. Clinical onset of SLE is often preceded by epitope spreading with development of antibodies to multiple nuclear antigens (Ref. 2). In addition, recruitment of inflammatory cells and mediators to susceptible target organs is required. Multiple genetic pathways predispose to development of SLE, some of which are shared by other autoimmune diseases; usually there are contributions from three or more susceptibility alleles in individual patients (Ref. 3). The disease may be triggered in genetically susceptible individuals by environmental factors, including microbial antigens, drugs, toxins and hormones. Because a single cause for SLE has not been identified, therapy has relied on global immunosuppression; however, this causes significant morbidity and mortality from unwanted side effects including infections, osteoporosis, infertility and premature atherosclerosis. More than a hundred single genetic defects affecting the immune system cause SLE-like disease in mice (Refs 3, 4, 5), suggesting that Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ Feinstein Institute for Medical Research, NS-LIJHS, Manhasset, NY 11030, USA. *Corresponding author: Anne Davidson, Feinstein Institute for Medical Research, NS-LIJHS, Autoimmune Laboratory, 350 Community Drive, Manhasset, NY 11030, USA. Tel: +1 516 562 3840; Fax: +1 516 562 2953; E-mail: [email protected] 1 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press there are many pathways that can be targeted therapeutically. The major challenge to assembling a new therapeutic armamentarium for SLE is the difficulty in conducting largescale clinical trials in this highly complex disease. In addition, because of the heterogeneity of the disease, targeting a particular immune pathway may not be equally successful for each patient. Furthermore, restoration of homeostasis may be difficult to achieve because insufficient or excessive cell activation can predispose to autoimmunity. For example, insufficient B-cell signalling can result in selection of autoreactive B cells into the naive B-cell repertoire (Ref. 6), whereas excessive B-cell signalling can result in the escape of autoreactive cells during antigenic stimulation (Ref. 7). In addition, many inflammatory mediators have pleiotropic effects that are dependent on the microenvironment and the cell activation state. Finally, synergistic combinations of drugs that allow smaller doses and therefore less toxicity need to be identified. The innate immune system as a target for SLE therapy The innate immune system comprises cells and soluble molecules that are the first responders to an immune insult. Receptors on cells of the innate immune system recognise microbial components and induce cellular activation and cytokine release that induce activation and proliferation of T and B cells. The innate immune system is also crucial for noninflammatory clearance of apoptotic material generated from normal cell turnover. Circulating natural antibodies, complement and other acute-phase proteins opsonise and help specialised cells to clear foreign material. A defect in these functions can give rise to autoimmunity. An emerging concept in the pathogenesis of SLE is that an excessive load of apoptotic particles containing nuclear antigens or of immune complexes containing autoantigens can overcome self-tolerance mechanisms and trigger autoimmunity (Refs 8, 9). Loss of tolerance may be due to excessive generation of such material, deficiencies of circulating ‘natural’ IgM antibodies, complement and other proteins that are involved in opsonisation and clearance, or alterations in thresholds for signalling of the innate immune response expert reviews in molecular medicine (Refs 10, 11, 12, 13). One mechanism for the pathogenicity of increased antigenic or apoptotic load is through activation of Toll-like receptors (TLRs) that recognise foreign and endogenous nucleic acids (Ref. 14). Four TLRs – TLR3, TLR7, TLR8 and TLR9 – expressed predominantly by antigen-presenting cells (APCs) and B cells belong in this category (Ref. 15). There has been much focus recently on TLR9 (which recognises bacterial CpG-rich DNA) and TLR7 (which recognises viral single-stranded RNA), which are normally sequestered inside endosomes away from circulating endogenous nucleic acids (Refs 15, 16). However, these endosomes are situated adjacent to phagosomes that take up apoptotic material. In addition, nucleic acids within immune complexes can be delivered to TLR-containing endosomes after cellular uptake via either cell-surface B-cell receptors (BCRs) or Fc receptors (Refs 17, 18). Blockade of this process underlies the therapeutic effect of antimalarial drugs in SLE patients (Ref. 19). Stimulation of TLR7 and TLR9 leads to transcription of genes encoding interleukin 6 (IL-6), IL-12, tumour necrosis factor a (TNF-a), type I interferons (IFNs) and other innate immune effectors (Refs 20, 21, 22) (Fig. 1). Polymorphisms of the TLR-induced transcription factor gene Irf5 are associated with SLE in humans, indicating the importance of this pathway in disease (Ref. 23). One important mechanism by which TLR ligation might contribute to SLE pathogenesis is by induction of type I IFNs. IFN-a is overexpressed in human SLE patients and accelerates SLE in humans and in some genetically predisposed strains of mice (Refs 24, 25) (see below). Other functions reported as a result of TLR engagement include blockade of suppressor cell function (Ref. 26), maturation of monocytes into macrophages and dendritic cells (Ref. 27), and inhibition of shedding of inducible costimulator (ICOS) ligand from B cells, resulting in increased T-cell help through ICOS (Ref. 28). TLR9 ligands can induce either inflammation or tolerance, depending on the mode and timing of delivery (Ref. 29). The recent in vitro finding that release of type I IFNs following activation of TLR9 ligands is dependent on the presence of the inflammatory mediator HMGB1 suggests a mechanism by which inflammation controls the outcome of TLR9 ligation (Ref. 30). The effect of Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 2 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine Immune complex containing nucleic acid IgG IgM HMGB1 Phagocytosis Endocytosis BCR Fc receptor RAGE dsRNA DNA TLR3 TLR9 b ssRNA Endosome c TLR7/8 d Adaptors e p38/JNK NF-κB IRF1 TNF-α IL-12 IL-6 Type 1 IFN g h f Complement a Apoptotic particle IRF7 IRF5 The innate immune system in systemic lupus erythematosus Expert Reviews in Molecular Medicine © 2008 Cambridge University Press Figure 1. The innate immune system in systemic lupus erythematosus. Immune complexes containing nucleic acid antigens and apoptotic bodies are internalised by B cells via the BCR and Fc receptor, by dendritic cells via the Fc receptor and by macrophages via Fc receptors and endocytosis. Complexes are directed to endosomes containing nucleic-acid-binding TLRs. Via recruitment of adaptor molecules, this leads to activation of transcription factors (such as p38, JNK, NF-kB, IRFs) that drive the production of inflammatory cytokines. Alterations in many different components of this pathway may induce or offer protection from SLE. (a) Immune complexes and apoptotic particles are cleared by various molecules including natural IgM, complement, DNases and serum amyloid P. Deficiencies of these molecules can cause SLE. (b) An extra copy of TLR7/8 (part of the Yaa locus) accelerates murine SLE. (c) TLR3 deficiency has no effect on murine SLE and TLR9 deficiency decreases anti-DNA antibodies but does not improve disease outcome. (d) TLR7 deficiency decreases anti-RNA antibodies and improves disease outcome in mice. (e) Polymorphisms of IRFs are associated with SLE. (f) TNF-a blockade can induce SLE but has also been shown to induce remission of established kidney disease. Thus its effects are stage specific. (g) IL-6 blockade delays disease in murine models. (h) Type I IFN signature is observed in active SLE patients. Deficiency of type I IFN receptor prevents disease in some SLE models but administration of type I IFN is protective in others. Polymorphism of IFN-receptor-associated signalling molecule Tyk2 is associated with SLE in humans. Abbreviations: BCR, B-cell receptor; HMGB, high-mobility group box 1; IFN, interferon; IRF, interferon regulatory factor; JNK, Jun kinase; RAGE, receptor for advanced glycation end product; TLR, Tolllike receptor. Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 3 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press TLR9 deficiency in murine models of SLE is strain dependent. Although the titres of anti-doublestranded DNA and nucleosome antibodies are decreased in autoimmune-prone TLR9-deficient mice (Ref. 31), protection has been observed in some mice (Ref. 32) but increased autoantibodies to RNA antigens and increased mortality have been observed in others (Ref. 33). By contrast, TLR7 clearly contributes to SLE pathogenesis. TLR7-deficient SLE-prone mice fail to mount antibodies to ribonuclear antigens and have a more moderate disease course than their wild-type counterparts (Refs 31, 34). Given this emerging knowledge, it has therefore been of much interest that the Ylinked Yaa gene – an accelerator of SLE in the BXSB male mouse – was found to be a reduplication of part of the X chromosome containing Tlr7 and Tlr8 (Ref. 35). NZW/BXSB mice express high titres of antibodies to RNA and have a marked expansion of the B-cell, monocyte and dendritic cell lineages that express TLR7 (Ref. 36). Immune complexes containing RNA are potent stimulators of TLR7 and TLR8, especially in the presence of IFN-a and can therefore amplify and perpetuate disease. Further complexity is added by the differential expression of TLR7, TLR8 and TLR9 in different cell types and their ability to crossregulate each other (Ref. 14). Oligodeoxynucleotide inhibitors of TLR signalling (ODNs) containing methylated DNA or DNA containing CCGG or TTAGGG motifs have recently been developed and inhibit IFN-a production in response to immune complexes or viruses (Ref. 15). Synthesis of inhibitors with dsDNA-like structure may be selective for autoreactive cells because these are preferentially taken up into anti-dsDNA-secreting B cells via the BCR (Ref. 37). Administration of ODNs to SLE-prone mice results in delay in both the onset and progression of SLE nephritis (Ref. 38). Interestingly, in a mouse model of SLE, an inhibitor of both TLR7 and TLR9 did not add clinical benefit to the effects of specific TLR7 or TLR9 inhibitors and had less effect on antidsDNA titres than TLR9 inhibition alone (Ref. 39). Since much of the effect of TLR9 ligation is mediated by IFN-a, inhibition of this cytokine is also currently being tested in human clinical trials. Another important component of the innate immune system is the Fc receptor family, which expert reviews in molecular medicine regulates responses to immune complexes (Ref. 40). Four different classes of human IgG Fc receptors have been identified, with differing affinities for IgG and for specific IgG isotypes and with differential expression on particular lymphoid cell types. FcRI, FcRIIA and FcRIIIA are activating receptors that are broadly expressed on cells of the haematopoietic lineage. FcRIIB is an inhibitory receptor expressed on all immune cells and is the only Fc receptor expressed on B cells. FcRIIB inhibits B-cell responses to immune complexes and helps terminate humoral immune responses once there is antibody excess over antigen. Expression of FcRIIB is constitutively low in several mouse models of SLE and the disease can be reversed in these mice by inducing only 40% more FcRIIB expression on B cells (Ref. 41). Polymorphisms of FcRIIB are associated with human SLE, but also with protection from infectious diseases (Ref. 42). In addition, humans with SLE fail to upregulate FcRIIB on memory and plasma cells, a defect that is partly genetically determined and partly acquired (Refs 43, 44). Complete absence of FcRIIB in mice leads to autoimmunity and SLE-like disease in a strain-specific manner (Ref. 45). Analysis of these mice has indicated that FcRIIB regulates B-cell tolerance in the periphery, acting as a brake on the differentiation of autoantibody-producing plasma cells (Ref. 46). FcRIIIA and its newly described mouse homologue FcRIV are intermediate-affinity receptors that recognise immune complexes but not monomeric IgG. These receptors are thought to be the activating receptors for immune complexes in vivo, because highaffinity Fc receptors are already occupied with monomeric Ig (Ref. 40). Polymorphisms of FcRIIIA that lower antibody-binding affinity are associated with susceptibility to SLE nephritis and with poorer responses to therapy with B-cell-depleting agents. Low-affinity binding FcRIIA polymorphisms have also been associated with SLE and with antiphospholipid syndrome (Refs 47, 48). These polymorphisms of activating receptors may result in decreased immune complex clearance. The relative expression of activating and inhibitory Fc receptors influences the outcome of immune-complex stimulation of cells, as does the isotype of the antibodies in the complexes. During inflammation, FcRIIB is Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 4 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press downregulated whereas activating Fc receptors are upregulated, resulting in activation of immune cells (Ref. 40). This may contribute to the failure of upregulation of FcRIIB on B cells in patients with active SLE. One proposed mechanism of action of intravenous immunoglobulin is that it upregulates FcRIIB, thus altering the balance of activating to inhibitory receptors (Ref. 49). This effect appears to be dependent on a sialic-acidbearing IgG glycoform that acts through an as yet unidentified receptor (Ref. 49). Further understanding of this pathway may lead to novel therapies for SLE based on modulating FcR function. Targeting activation of the acquired immune response in SLE Triggering of the innate immune system by exposure to exogenous antigen is crucial for the activation of antigen-specific T and B cells and their subsequent clonal proliferation and differentiation into memory cells. Pathogenic autoantibodies in SLE are isotype switched and somatically mutated – functions dependent on T-cell help (Ref. 50). Self-tolerance of T cells is maintained by many mechanisms, including sequestration from self-antigen, deletion of highly self-reactive cells in the thymus, and peripheral regulation either by APCs that have not been activated by innate mechanisms or by other regulatory cell subsets (Refs 51, 52). Very little is known about the antigenic specificity of T cells in SLE. Therefore, T-cell-directed therapies for SLE have focused on non-antigenspecific pathways. Although the specificity of the T-cell response is provided by the interaction of the major histocompatibility complex (MHC)– peptide complex with the T-cell receptor (TCR), T-cell activation requires a second set of costimulatory signals between T cells and APCs. The last decade has seen the identification of many costimulatory receptor – ligand pairs that are potential therapeutic targets. CD28– B7 family members CD28 is constitutively expressed on T cells and is upregulated upon T-cell activation. CD28 engagement by its ligands CD80 and CD86 (B7-1 and B7-2) amplifies signals through the TCR and stabilises the immunological synapse, thus lowering the threshold for naive T-cell activation, and facilitating entry into the cell cycle and secretion of IL-2 (Refs 53, 54). expert reviews in molecular medicine Engagement of the TCR in the absence of CD28 costimulation may lead to cell death or induction of anergy (Refs 55). This maintains tolerance to self-antigens that are usually chronically encountered in the absence of costimulatory signals. The CD28-homologous molecule CTLA4 has much higher affinity for CD80 and CD86 and serves as a negative regulator of T-cell function. CTLA4, unlike CD28, signals through CD80 and CD86 to induce several regulatory molecules including indoleamine 2,3-dioxygenase (IDO), an enzyme that helps maintain tolerance through alterations in tryptophan metabolism (Refs 56, 57). A recently identified polymorphism of CTLA4 is associated with autoimmunity in humans (Ref. 58). Blockade of CD28 is achieved by administration of the extracellular domain of CTLA4 fused to immunoglobulin heavy chain (CTLA4Ig, Abatacept) (Ref. 54). CTLA4Ig acts both as a competitive antagonist of B7– CD28 interactions and as an inducer of regulatory pathways in APCs. CTLA4Ig may also alter expression of adhesion molecules and chemokine receptors, resulting in inhibition of traffic of inflammatory cells to target organs (Ref. 59), and prevents the upregulation of ICOS (Ref. 36). However, under some circumstances, administration of CTLA4Ig might be detrimental. For example, although CTLA4Ig can induce T-cell deletion and anergy, it may prevent tolerance induction in circumstances where signalling through CTLA4 is necessary to establish active tolerance (Ref. 60). CTLA4Ig and its higher-affinity mutant LEA29Y (Belatacept) have been successfully used in patients with psoriasis, rheumatoid arthritis and transplantation (Refs 54, 61). In mice, CTLA4Ig prevents the onset of SLE (Ref. 62) but does not reverse active inflammation (Ref. 63). For this reason, combination therapies have been tested. Administration of CTLA4Ig with concomitant CD40 or CD40L blockade to SLE-prone mice is a highly synergistic regimen that prevents the onset of SLE for many months after a short course of treatment. This is due to long-lasting blockade of T-cell help for autoreactive B cells (Ref. 64). Dual treatment with a single dose of the alkylating agent cyclophosphamide and continuous CTLA4Ig is highly synergistic for the treatment of active SLE nephritis in mice and induces complete remission of histological Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 5 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine changes in the kidney. The mechanism for this effect is not completely understood, but includes depletion of activated T and B cells, together with altered renal chemokine and chemokine receptor expression, and consequent dampening of the inflammatory response to glomerular immune-complex deposition (Ref. 65). Clinical trials of Abatacept in active SLE and in combination with cyclophosphamide in SLE nephritis have been initiated. B7– CD28 interactions are essential for activation of naive T cells, but have a lesser role in regulating effector responses and responses outside secondary lymphoid organs. Expression of other B7 family members allows regulation of effector T cells at peripheral sites (Ref. 66) (Fig. 2). ICOS is a CD28-like molecule that is expressed on activated and memory T cells and preferentially induces IL-4 and IL-10 production (Ref. 67). ICOS is highly expressed on IL-10-expressing effector cells (Ref. 68) and on IL-21-secreting CXCR5-positive follicular T-helper (Th) cells located in the apical zone of germinal centres where somatic mutation occurs (Ref. 69). Ligation of ICOS results in upregulation of CD40L on T cells, which costimulates immunoglobulin synthesis and differentiation of B cells to memory and plasma cells (Ref. 70). Dramatic defects of the humoral immune response occur in ICOS-deficient mice and humans as well as defects in IL-10 and IL-17 production (Refs 71, 72). Antagonism of ICOS during the effector phase of Th2-mediated disease attenuates inflammation in murine models (Ref. 72). The ICOS ligand B7RP-1 is expressed on B cells and macrophages and in inflamed tissues. ICOS expression is increased on T cells from SLE-prone mouse strains, particularly mice that bear a Yaa mutation (Ref. 36) and on T cells in SLE patients (Ref. 73). Coinhibition ? –– BTLA –– PD-1 CTLA4 –– –– Costimulation CD28 ++ ICOS ++ ? ++ TCR/CD3 ++ B7.2 B7H B7-H3 MHC T cell APC B7-H4 HVEM PDL1/2 B7.1 Members of the CD28–B7 family mediate both costimulation and coinhibition Expert Reviews in Molecular Medicine © 2008 Cambridge University Press Figure 2. Members of the CD28 –B7 family mediate both costimulation and coinhibition. Two signals are required for T-cell activation. Signal 1 is mediated through interaction of the T-cell receptor (TCR) with MHC/ peptide complex shown in blue. Signal 2 is mediated by non-antigen-specific interactions such as that of B7 with CD28. CD28-like receptors are shown on the surface of the T cell and B7-like ligands (blue) are shown on the surface of the antigen-presenting cell (APC). Receptors shown in green transduce a positive (costimulatory) signal to the T cell. Receptors shown in red transduce a negative (coinhibitory) signal to the T cell. Not all the receptors have been identified (indicated by ?). Abbreviations: BTLA, B and T lymphocyte attenuator; HVEM, herpesvirus entry mediator; ICOS, inducible costimulator; MHC, major histocompatibility complex. Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 6 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews The pathogenic role of ICOS has recently been highlighted by the development of B-cell autoimmunity in mice deficient in roquin, a repressor of ICOS expression (Ref. 74), and by studies showing that blockade of ICOS ligand prevents and treats disease in NZB/W SLEprone mice (Ref. 75). Clinical trials of ICOS blockade in humans with SLE are now under way. The receptor programmed death 1 (PD-1), which is expressed on both B and T cells, binds to two ligands: PDL1, which is widely expressed on many tissues and PDL2, which is expressed mainly on activated monocytes. Like CTLA4, PD-1 transduces a negative signal into the cell and helps to maintain peripheral tolerance (Ref. 76). Studies in mouse models of multiple sclerosis and diabetes have shown that this effect is mediated via the interaction of PD-1 with PDL1 but not with PDL2 (Refs 77, 78). PD-1-deficient mice develop spontaneous strain-specific autoimmunity including SLE (Ref. 79) and recently a PDCD1 polymorphism associated with human SLE has been identified (Ref. 80). Several other members of the CD28 – B7 family have been characterised. B and T lymphocyte attenuator (BTLA), like CTLA4 and PD-1, is a negative regulator of T-cell responses and its deficiency results in increased susceptibility to induced autoimmunity. BTLA is unusual because it binds herpesvirus entry mediator (HVEM), a member of the tumour necrosis factor receptor family (Refs 81, 82). B7-H3 and B7-H4 bind to unidentified receptors (Ref. 83). Little is currently known about the role of these molecules in autoimmune disease pathogenesis; however, these molecules appear to have their effects only at low antigen doses. The TNF –TNFR family Multiple members of this family mediate costimulation of T cells and B cells, with 4-1BB, CD40L and B-cell activating factor (BAFF) being most relevant to SLE therapies. 4-1BB The engagement of the T-cell costimulatory receptor 4-1BB (CD137) paradoxically prevents germinal centre formation in a T-cell-dependent fashion. In SLE-prone mice, three doses of an agonistic anti-4-1BB antibody confers prolonged inhibitory effects on autoantibody production starting 1–2 weeks after antibody in molecular medicine administration (Ref. 84). In the chronic graftversus-host (CGVH) model of SLE, administration of the anti-4-1BB antibody resulted in rapid depletion of donor T cells by activation-induced death (Ref. 85). By contrast, in the NZB/W model, CD4þ T cells were not depleted, but cytokine secretion by these cells was markedly diminished (Ref. 84). Transfer of either activated T cells or APCs from untreated mice overcame the T-cell anergy and precipitated disease. Another hypothesis for the activity of anti-4-1BB is that T-regulatory cells (T regs) are generated (Refs 84, 86). Although a regulatory population of CD8þ cells has been described, it is clear that the antibody still mediates tolerance in CD8-deficient mice (Ref. 84). Finally, production of IFN-g has been shown to enhance tolerance in the Murphy Roths Large (MRL)/lpr model (Ref. 87) but not in the CGVH model, suggesting that IFN-g is not essential for tolerance induction (Ref. 85). This system is interesting because it shows that long-lasting restoration of tolerance can be achieved in mice with spontaneous SLE in which B and T cells are continuously being activated. Further studies in this system may reveal the mechanisms for maintenance, loss and restoration of tolerance in autoimmunity. CD40L CD40L is an important costimulatory molecule, whose expression on activated T cells is regulated by CD28 and by ICOS (Ref. 83). Engagement of CD40 on APCs by CD40L synergises with TLR signalling and results in increased expression of MHC and B7 molecules and secretion of IL-12, thus enhancing their antigen-presenting function (Ref. 88). Engagement of CD40 on B cells delivers a survival signal and is essential for formation of the germinal centre and for reactivation of memory B cells (Refs 89, 90). Absence of CD40 or CD40L in humans results in a profound humoral immunodeficiency (Ref. 91). Antibodies to CD40L prevent onset and delay progression of SLE in mouse models (Ref. 92), but two Phase II human clinical trials failed because of the unexpected development of thrombotic events in some of the treated patients (Ref. 93). Although one of the anti-CD40L trials failed to show clinical efficacy at the dose used (Ref. 94), some of the SLE patients treated with a different anti-CD40L Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 7 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press antibody showed clinical improvement (Ref. 95). Mechanistic studies in these patients revealed that despite unaltered total B-cell numbers, treatment diminished the number of autoantibody-producing B cells (Refs 95, 96, 97). It has been suggested that the effect of antiCD40L is due to specific B-cell unresponsiveness rather than T-cell anergy, because tolerance cannot be broken in mice by transfer of pathogenic T cells (Ref. 98). However, in humans with SLE, B cells producing anti-DNA antibody reappeared after cessation of anti-CD40L, indicating that tolerance had not been established (Ref. 96). A striking therapeutic effect can be observed in mouse models when antiCD40L is combined with CTLA4Ig. This combination, given for two weeks to SLE-prone mice, induces a prolonged effect on disease when given early in the course (Ref. 63), and, more importantly, maintains B-cell tolerance to autoantigens without preventing immune responses to exogenous antigens (Ref. 64). However, this combination is not effective in the setting of active nephritis and requires the addition of a single dose of cyclophosphamide, which kills activated effector cells (Ref. 65). Although anti-CD40L antibodies are not safe for use in humans, antibodies to CD40 have been recently developed for human use and appear to be safe for the treatment of B-cell tumours (Ref. 99). This may allow for a renewed attempt at CD40 blockade in human SLE. BAFF The TNF family member BAFF is a B-cell survival factor expressed by many different cell types that binds to three different BAFF receptors (TACI, BCMA and BAFF-R), which are variably expressed on the B-cell surface during development (Refs 100, 101). BAFF-R and TACI are the predominant receptors on most mature B cells, except for plasma cells, which express BCMA (Refs 102, 103). APRIL, a closely associated ligand of BAFF, binds to TACI and BCMA and to proteoglycans such as CD138 on the plasma cell surface; CD138 serves as a coreceptor to enhance binding of APRIL to BCMA (Ref. 104). Maintenance of peritoneal cavity B1 B cells is BAFF independent (Refs 105, 106) and germinal centre B cells are only partially dependent on BAFF. Thus BAFFdeficient mice can still develop autoimmunity, although they do so in a much delayed fashion expert reviews in molecular medicine (Refs 107, 108). BAFF transgenic mice on a nonautoimmune genetic background develop a form of SLE that is due to production of autoantibodies by marginal zone and B1 cells and this depends on B-cell signalling through TLRs but does not require T cells (Ref. 109). BAFF levels may control B-cell selection because competition for BAFF limits selection of autoreactive B cells into the mature compartment. Depletion of B cells results in excess production of BAFF, which may then decrease the stringency for B-cell selection (Refs 110, 111). It remains unclear whether the increased sensitivity of autoreactive B cells to survival signals mediated through BAFF and APRIL is applicable to memory cells, plasmablasts or plasma cells (Ref. 112). Patients with SLE and other autoimmune diseases have increased serum levels of BAFF (Refs 113, 114) and in murine SLE nephritis, BAFF is also found locally at the site of inflammation where it is made by activated macrophages and dendritic cells (Ref. 207). Activated macrophages express BAFF receptors and thus may mediate their own survival in an autocrine fashion (Ref. 115). BAFF blockade has been achieved either with a monoclonal anti-BAFF antibody or with soluble BAFF receptors. BAFF-R-Ig selectively blocks BAFF, whereas TACI-Ig blocks both BAFF and APRIL, and therefore is able to deplete plasma cells that are dependent on APRIL– BCMA interactions. Despite these differences, both selective and nonselective BAFF blockers prevent SLE in mouse models (Ref. 106), indicating that BAFF is more important than APRIL in SLE pathogenesis. BAFF blockade depletes B cells by 50% but does not significantly decrease the titres or affinity of autoantibodies, nor does it prevent T-cell activation. By significantly decreasing B-cell numbers and spleen size, BAFF blockade results in a decrease in the total number of activated T cells and monocytes, thus decreasing the total inflammatory load and the capacity of inflammatory cells to migrate to the kidney. The combination of BAFF blockade and CTLA4Ig, but not either drug alone, can induce remission of SLE in NZB/W mice by mechanisms that are not fully delineated (Refs 106, 116). A human clinical trial of the anti-BAFF antibody Belimumab in moderately active SLE showed only modest clinical effects, Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 8 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press with failure to meet initial efficacy end points. However, long-term follow up and post-hoc analysis of patients that were serologically active at initiation has shown a greater sustained improvement in treated patients than in placebo-treated controls (Ref. 117). More extensive trials of this agent are in progress. TACI-Ig is also entering clinical trials in SLE. B-cell modulation and depletion SLE is characterised by the production by B cells of high-affinity pathogenic autoantibodies that mediate tissue injury. In addition, B cells have other important functions that can contribute to disease pathogenesis. Activated autoreactive B cells that take up autoantigen through their antigen receptor can function as APCs and serve to diversify the epitopes of self-antigen presented to autoreactive T cells. B cells also produce cytokines involved both in lymphoid regulation and in inflammatory processes (Ref. 118). Because autoreactive B cells play a role in both the inductive and the effector arms of autoimmune disease, there has been much interest in B-cell depletion or modulation as a treatment strategy for autoimmune disease. One such strategy has been the use of anti-CD20 antibodies. CD20, a transmembrane protein with four subunits, is upregulated on most B cells past the pre-B-cell stage but is absent on plasma cells (Refs 119, 120). Anti-CD20 monoclonal antibodies successfully deplete peripheral human B cells for periods ranging from three months to more than one year through mechanisms involving Fc-receptor dependent killing, complement-dependent killing and antibody-dependent apoptosis (Refs 121, 122). Studies in mice show that the kinetics of B-cell depletion varies between different B-cell compartments with 90% follicular B cells in the spleen being depleted within two days of antiCD20 monoclonal antibody administration but less effect on marginal zone B cells. The peritoneal cavity (particularly B1) and the germinal centre B-cell compartments demonstrate the greatest relative resistance to anti-CD20 monoclonal antibody treatment although marginal zone, germinal centre and peritoneal B1 B cells express comparable levels of CD20 and appear to have access to the antibody. These differential sensitivities depend partly on the number and localisation of expert reviews in molecular medicine mononuclear phagocytes within tissues that mediate antibody-dependent cell-mediated cytotoxicity (Refs 122, 123, 124). Hence, location and microenvironment influence the extent of B-cell depletion (Ref. 123). Because B-cell depletion causes a compensatory increase in BAFF levels, blockade of BAFF synergises with anti-CD20 monoclonal antibody to enhance B-cell depletion (Ref. 123). A new approach that may avoid the need for two drugs is the use of a depleting anti-BAFF-R antibody. This antibody blocks the effects of BAFF and also depletes BAFF-R-bearing cells. However, like anti-CD20, this antibody does not deplete long-lived plasma cells that no longer express BAFF-R (Ref. 125). Preliminary clinical experience with a mouse – human chimaeric anti-CD20 antibody (Rituximab) in patients with SLE (Ref. 126) and other autoimmune diseases has been encouraging (Refs 127, 128, 129), with rapid resolution of symptoms in some patients that depends on B-cell depletion but not on depletion of autoantibodies. Autoantibodies continue to be produced because plasma cells do not express CD20. These studies confirm the role of B cells as important effector cells independently of their ability to produce antibodies. The degree of B-cell depletion is affected by FcRIIIA genotype; there is less depletion in patients bearing the low-affinity allele, which is also a SLE-susceptibility allele (Ref. 130). In addition, the rapid induction of human antichimaeric antibodies (HACA) has prevented B-cell depletion in some SLE patients. A fully human anti-CD20 is now available and may help solve this problem. In some patients, remissions are long-lived and returning B cells are predominantly of the transitional phenotype with low numbers of memory B cells (Ref. 131). The reappearance of memory B cells is also associated with earlier relapse in rheumatoid arthritis patients treated with anti-CD20 (Ref. 132). Long-term remissions have been associated with the absence of antibodies to extractable nuclear antigens (Ref. 133). Progressive multifocal leukoencephalopathy has been observed in two SLE patients that received more than one course of anti-CD20 together with other immunosuppressive therapy. Most of the reports of the efficacy of anti-CD20 in SLE have been uncontrolled case series in small numbers of treatment-resistant patients in which the Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 9 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press drug has been combined with other therapies including high doses of corticosteroids and cyclophosphamide. A placebo-controlled Phase III trial of anti-CD20 in SLE patients is now in progress. CD22 is a member of the sialoadhesin (siglec) family of adhesion molecules that is coexpressed with the B-cell receptor on mature B cells and up to the late germinal centre stage (Refs 129, 134, 135). CD22 has both immunoreceptor tyrosine activation motif (ITAM) and immunoreceptor tyrosine inhibitory motif (ITIM) domains in its intracellular domain and can act both as a negative regulator of BCR signalling and as a costimulatory molecule, depending on the context in which it is activated (Ref. 136). Because of this complexity, the precise effect of CD22 upon BCR signalling in B cells at the various developmental stages is not fully elucidated. B cells from CD22-deficient mice have a shortened lifespan and the mice have reduced circulating B-cell numbers; however, these mice also have hyper-reactive B cells and may develop autoantibodies with age (Ref. 137). Epratuzumab, a humanised monoclonal antiCD22 antibody, can mediate antibodydependent cellular cytotoxicity and has been used to treat human B-cell malignancies (Ref. 138). The rationale for using anti-CD22 in human autoimmunity is based on the notion that modulating B-cell function may be safer than therapies that deplete B cells. However, given the complexity of CD22 signalling in B cells and the ability of this molecule to mediate functions both in a ligand-dependent and a ligand-independent fashion, it is difficult to predict what the effect of an anti-CD22 antibody will be in humans with autoimmune disease. The first clinical trial of epratuzumab in 14 SLE patients with mild to moderately active SLE has just been reported (Ref. 139). Treatment with anti-CD22 appeared to be safe and resulted in modest B-cell depletion in some of the patients. Serum levels of IgG and C3 were not altered, and autoantibody levels either increased or stayed the same. Although clinical efficacy could not be assessed in this study, improvements in BILAG (British Isles lupus assessment group) scores were reported. Mechanistic studies of patients in this clinical trial showed preferential targeting of CD27negative naive or transitional B cells that have expert reviews in molecular medicine high levels of cell surface CD22 but no decrease in memory B cells or plasma cells (Ref. 140). Cell-surface CD22 levels were downregulated by epratuzumab. Cell culture experiments further showed that epratuzumab modulated the abnormal proliferative response of SLE B cells to TLR or CD40 ligation. An antiproliferative effect was also observed in vitro when human B-cell lines were immobilised and stimulated with anti-IgM (Ref. 141). Further studies will be needed to determine whether these modest B-cell effects result in a measurable clinical effect in controlled trials. Depletion of autoantibodies or specifically of autoantibody-producing B cells is another approach that is currently in development. Abetimus sodium is a compound consisting of four double-stranded 20-mer ODNs that binds to anti-dsDNA antibodies and clears them from the serum. In mice, this drug has been shown to decrease splenic autoantibody-producing B cells, but it is not clear whether this also occurs in humans. Although the drug depletes anti-DNA antibodies in SLE patients by 30%, two Phase III clinical trials have failed to reach their primary endpoints of decreased time to renal flare and clinical benefit was demonstrated only using post-hoc analysis of a patient subset with high-affinity abetimusbinding antibodies at trial initiation (Ref. 142). Further studies of this agent are in progress. Other compounds directed specifically at autoantibody-producing B cells have been developed and are described in a recent review (Ref. 143). Cytokines in SLE – pleiotropic activities may complicate treatment Cytokine inhibition has been used successfully to treat several autoimmune and inflammatory diseases but has not yet been applied to the treatment of SLE. One reason is that a number of pro-inflammatory cytokines are also required for the active maintenance of tolerance and their blockade may exacerbate the loss of tolerance to ubiquitous antigens. For example, TNF-a blockade enhances the production of SLE-related autoantibodies. Crosstalk between innate immune system signals and cytokines can be dysregulated in a systemic inflammatory environment and this may result in proinflammatory functions of cytokines that are usually anti-inflammatory. For example, IL-10 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 10 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews usually signals through STAT-3 but in the presence of type I IFNs it switches to the proinflammatory STAT-1 (Refs 144, 145). In addition, upregulation of costimulatory receptors and integrins, and alteration in the ratio of activating to inhibitory Fc receptors, can alter the cellular response to cytokines. IL-6 IL-6 is a multifunctional cytokine that is critical for the development and functioning of many different cells and plays a crucial role in immune responses and inflammation (Refs 146, 147). In the setting of activation of the innate immune system, IL-6 dampens T reg function (Ref. 26). Conversely, IL-6 has a regulatory role in the resting immune system as it has been shown to maintain anergy of resting B cells chronically exposed to self-antigens (Ref. 208). Excessive secretion of IL-6 is found in many autoimmune diseases (Ref. 148) including SLE (Ref. 149), where it induces plasma cell and effector T-cell differentiation (Refs 26, 150). A critical role for IL-6 in the pathogenesis of SLE was demonstrated by the beneficial effects of IL-6 receptor blockade and the exacerbating effect of IL-6 in NZB/W F1 mice (Refs 146, 151, 152). A Phase I clinical study using an anti-IL6R monoclonal antibody (MRA) in SLE has been completed but the development of dosedependent neutropaenia may complicate the use of this agent in human SLE (Ref. 153). TNF-a TNF-a is a pleiotropic cytokine that can mediate both cell survival and cell death (Ref. 154). The importance of this cytokine in maintaining the balance of the immune system is highlighted by acceleration of autoimmunity in a TNF-adeficient background (Refs 155, 156) and the triggering or maintenance of autoimmunity in the setting of excess TNF-a (Refs 157, 158, 159). TNF-a antagonists have a remarkable therapeutic effect in a number of autoimmune diseases including rheumatoid arthritis and Crohn disease (Refs 160, 161, 162). However, TNF-a blockade appears to exacerbate certain autoimmune diseases (Ref. 163) and has resulted in the emergence of new autoimmunity in up to 50% of treated patients with the development of antinuclear antibodies and even clinical SLE (Ref. 164). By contrast, TNF-a is known to be pathogenic in mouse models of in molecular medicine nephritis and is produced by intrinsic renal cells as a result of exposure to inflammatory cytokines including IL-1 (Ref. 165). Based on these findings, successful anecdotal use of TNF-a blockade has been reported in a small number of patients with refractory SLE nephritis (Ref. 166). Not surprisingly, autoantibody titres increased in the treated SLE patients, confirming that some features of autoimmunity are exacerbated by this approach. One hypothesis for this effect is that TNF-a blockade increases the load of apoptotic cells (Ref. 167). One group found increased expression of TNFRII on CD4þ CD25þ T regs of SLE patients. Signalling through this receptor resulted in loss of suppressive function of T regs that could be reversed by IL-2 supplementation (Ref. 168). Other hypotheses include the induction of IFN-a following TNF blockade and the requirement for TNF-a in negative regulation of activated T and B cells. Since TNF-a is made as a soluble and a membrane protein and can bind to two different receptors with different functions (Refs 169, 170, 171), there has been recent interest in selective TNF-a blockade as a strategy for a safer and more specific therapeutic response (Refs 163, 172). Type I interferons Type I IFNs made by plasmacytoid dendritic cells are implicated in the pathogenesis of SLE (Refs 173, 174). Their therapeutic use can induce SLE-like syndromes, and peripheral blood mononuclear cells from a subset of patients with SLE display increased expression of IFN-a-regulated genes (Refs 175, 176). IFN-a promotes the differentiation of monocytes into dendritic cells, induces differentiation of CD8þ T cells and, together with IL-6, induces differentiation of B cells into plasma cells. However, the role of IFN-a in autoimmunity is pleiotropic and absence of IFN-a or its receptor has had opposite effects in different mouse models of SLE (Ref. 177). The explanation for this might lie in genetic differences and/or in the different effects of IFN-a on resting and activated dendritic cells. In vitro experiments have shown that triggering of TLR9 in naive peripheral blood mononuclear cell cultures results in an IFN-a-dependent inhibition of inflammatory Th1 cytokine secretion upon subsequent activation (Ref. 178). For this reason, type I IFN therapy has been used to treat autoimmune diseases such as multiple Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 11 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press sclerosis. However, if type I interferons are administered to activated dendritic cells, proinflammatory cytokines are generated (Ref. 179). This suggests that the outcome of treatment with IFN-a antagonists in patients may be stage dependent and may not always be predictable. In addition, the recent finding that the interferon signature can be elicited in human monocytes in the absence of IFN-a by ligation of activating Fc receptors, together with blockade of inhibitory Fc receptors, suggests that there are multiple pathways for induction of inflammation by immune complexes (Ref. 180). Early clinical trials of anti-IFN antibodies are in process and should more definitively define the pathogenic role of this group of cytokines in SLE. IL-10 IL-10 is increased in patients with active SLE and correlates with disease activity (Refs 181, 182). Although IL-10 is classically considered an anti-inflammatory cytokine, its role in SLE appears to be an inflammatory one. In the presence of IFN-g and immune complexes, there is attenuation of the suppressive functions of IL-10 mediated by a decrease in expression of the IL-10 receptor and decreased activation of JAK-1 (Ref. 183). In the presence of IFN-a there is a gain of proinflammatory function of IL-10 leading to STAT-1-dependent expression of genes that are normally induced by IFN-g. In NZB/W mice, continuous administration of IL-10 accelerated onset of renal disease and treatment with an anti-IL-10 monoclonal antibody delayed disease onset (Ref. 184). By contrast, in NZM2410 mice, IL-10 was protective when administered early in disease (Ref. 185). Similarly, in MRL/lpr mice, IL-10 deficiency resulted in a more severe disease (Ref. 186). The differences between these models may be related to the amount of concomitant inflammation at the time of IL-10 administration or depletion. In a pilot open-label short-term study of a mouse anti-IL-10 monoclonal antibody in a small number of active SLE patients, disease improvement was evident at 2 months, with continued responses over 3–6 months (Ref. 187). Interferon g IFN-g accelerates disease in SLE-prone NZB/W mice whereas treatment with anti-IFN-g is expert reviews in molecular medicine protective (Refs 188, 189). IFN-g – / – MRL/lpr mice were also protected from early death with a reduction in the severity of glomerulonephritis. These studies highlight the importance of IFN-g in accelerating SLE development, presumably by increasing MHC expression and autoantigen presentation to otherwise quiescent nontolerant anti-self T cells, and also by promoting local immune and inflammatory processes (Ref. 190). By contrast, results in STAT-4- and STAT-6-deficient SLEprone mice showed surprisingly that a decrease in IFN-g and an increase in IL-4 accelerated SLE nephritis even though autoantibody titres were diminished (Ref. 191). This may be due to early regulatory effects of IFN-g on the development of Th17 effector cells. IL-17-secreting cells appear to be critical effector cells in rheumatoid arthritis and multiple sclerosis (Ref. 192), but very little is known as yet about the role of this cytokine in SLE. The inflammatory process in SLE nephritis Proliferative glomerulonephritis is the most severe form of SLE nephritis and is characterised by mesangial proliferation and infiltration of the kidney parenchyma by inflammatory cells. This process is initiated by deposition of antibodies in the renal glomeruli but it is increasingly recognised that this is not sufficient for renal inflammation to occur. In NZB/W SLE-prone mice deficient in activating Fc receptors, autoantibody and complement deposition in glomeruli do not result in renal damage (Ref. 193). Subsequently it was shown that the important Fc-receptor-bearing cells are of haematopoietic origin and that circulating monocytes bearing the activating receptors are sufficient to restore disease (Ref. 194). In SLE patients, polymorphisms of Fc receptors have been associated with the susceptibility to renal disease (Ref. 48). Other downstream effector pathways including chemokines and cell death molecules are also required to mediate kidney damage. By contrast, in the MRL/lpr model, interstitial inflammation can occur in the kidney without immune-complex deposition and in the absence of Fc receptors, perhaps indicating that endothelial activation is sufficient to initiate inflammatory cell migration into target organs in this mouse (Refs 195, 196). A model for the effector pathways involved in renal inflammation is shown in Figure 3. Microarray Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 12 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine analysis of human SLE biopsies has shown considerable heterogeneity in gene expression between patients. Infiltration by B cells and myeloid cells was observed in some patients, as was evidence of fibrosis. As observed in peripheral blood specimens, some patients also had a type I IFN signature in the kidney (Ref. 197). Invasion of the glomerulus and the interstitium by inflammatory cells involves different Mechanism 1 Immune complex deposition IgG chemokines and receptors. In particular, CCR2 and CCR5 mediate glomerular cell invasion whereas CCR1 mediates interstitial invasion (Refs 198, 199). Studies of chemokine expression in the kidneys of NZB/W mice have shown that a limited panel of chemokines is expressed in the early stages of nephritis. The onset of proteinuria coincides with expression of CCR2 and CCR5 ligands and with infiltration and activation of Therapy C3 Fc-receptor Costimulatory blockade TLR antagonists Complement inhibitors A 2 Endothelial activation Upregulation of selectins and integrins B MØ 3 Leukocyte adhesion Inflammatory chemokines Chemokine inhibitors 4 Local chemokine release CCL2, CCL5, CCL8, CCL20, CXCL13, others 5 Chemokine-enhanced cell infiltration 6 Inflammatory cytokines 7 Proaptotic factors (e.g. TGF-β, TNF-α, NO) Upregulation of chemokine receptors CTX/costimulatory blockade B-cell depletion T cell B cell Cytokine antagonists Transmigration Caspase inhibitors INOS inhibitors 8 Irreversible renal death The events that lead to renal failure in systemic lupus erythematosus Expert Reviews in Molecular Medicine © 2008 Cambridge University Press Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ Figure 3. The events that lead to renal failure in systemic lupus erythematosus. Disease is initiated by deposition of immunoglobulin and complement on the glomerular basement membrane. This is followed by an inflammatory cascade that involves engagement of activating Fc receptors by circulating monocytes, endothelial activation, chemokine secretion, recruitment of activated lymphocytes and finally release of proapoptotic factors that result in irreversible renal cell death. Potential therapies (shown in red) may target this cascade in a stage-specific manner. Abbreviations: B, B cell; T, T cell; MØ, macrophage; NO, nitrous oxide; TGF-b, transforming growth factor b; TNF-a, tumour necrosis factor a. 13 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine interstitial macrophages that secrete IL-1 and other inflammatory molecules. With progression of disease, there is extensive spreading of the inflammatory response to include multiple chemokines and cytokines (Ref. 207). These data suggest that it may be difficult to induce remission of active nephritis with single chemokine or cytokine inhibitors but that such reagents may be useful once most activated cells have been purged from the kidneys. Furthermore, these studies predict stage-specific therapies for SLE nephritis. For example, CCR2 and CCR5 blockade may be useful for treating the early stages of glomerular disease whereas IL-1 or TNF-a blockade may be useful for antagonising the proinflammatory effects of activated infiltrating macrophages. Since upregulation of CCR1 occurs only in mice with established proteinuria, CCR1 blockade may be effective for treatment of late- rather than earlystage disease. Consistent with this result, a CCR1 antagonist does not affect systemic immune activation or glomerular damage in SLE-prone Genetic susceptibility MRL/lpr mice but slows progression of interstitial renal disease and fibrosis (Ref. 198). As more is learned about the inflammatory process in the SLE kidney, application of therapies to prevent renal cell infiltration are likely to be tested. Clinical implications and research in progress Maintenance of immune homeostasis requires a finely tuned immune system that needs the flexibility to respond quickly to pathogens but does not mount immune responses to selfantigens. Both immunodeficiency and exaggerated immune responses can predispose to autoimmunity. In addition, cytokines and cell receptors that are balanced to maintain tolerance in the setting of a resting immune system can mediate inflammatory responses when the immune system is activated. Multiple defects of immune tolerance can lead to SLE and predisposing causes can include both immunodeficiency in the resting immune Trigger Initiation Progression End organ damage Goal Prevention Remission Flare prevention Re-establish tolerance Supportive therapy Target Naive B cells and T cells Activated cells Inflammatory mediators Memory cells Plasma cells Fibrosis Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ Therapies for systemic lupus erythematosus depend on the stage of disease Expert Reviews in Molecular Medicine © 2008 Cambridge University Press Figure 4. Therapies for systemic lupus erythematosus depend on the stage of disease. The various stages of SLE consisting of a pre-disease period followed by flares and remissions, are associated with differences in the state of immune activation which may require different therapeutic approaches. 14 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine Table 1. Common mouse models of systemic lupus erythematosus used for therapeutic studies Model (Refs 200, 202) Defect Major disease manifestations Clinically relevant autoantibodies MRL/lpr male and female Susceptible genetic background Deficiency of Fas Skin disease Arthritis Vasculitis CNS disease Nephritis Anti-DNA Anti-Sm Rheumatoid factor Antiphospholipid NZB male and female Susceptible genetic background Haemolytic anaemia Anti-RBC ANAs BXSB male Susceptible genetic background Yaa gene results in TLR7/8 overexpression Nephritis Anti-DNA Antiphospholipid Anti-Sm/RNP NZB/W female Susceptible genetic background Nephritis Anti-DNA Antiphospholipid NZW/BXSB male Susceptible genetic background Yaa gene results in TLR7/8 overexpression Nephritis Thrombocytopenia Antiphospholipid syndrome Anti-DNA Antiphospholipid Antiplatelet Anti-Sm/RNP NZM2410 male and female Inbred from NZB/W Nephritis (glomerulosclerosis) Antinucleosome Anti-DNA NZM2328 female Inbred from NZB/W Nephritis Anti-DNA Chronic GVHD Induced by transplant of MHC mismatched lymphocytes Nephritis Anti-DNA Abbreviations: ANAs, antinuclear antibodies; CNS, central nervous system; GVHD, graft-versus-host disease; MHC, major histocompatability complex; TLR, Toll-like receptor; RBC, red blood cell; RNP, ribonucleoprotein; Sm, Smith antigen. system and excessive immune responses in the activated immune system. For this reason, restoration of immune homeostasis in SLE patients poses many challenges. It is increasingly recognised that therapies for active disease, characterised by lymphoid cell proliferation and production of multiple inflammatory mediators, may need to be different from therapies that prevent disease flares or those that might eventually prevent disease onset in genetically predisposed individuals (Fig. 4). Despite these difficulties, many new drugs are being developed for the treatment of SLE and clinical trials are in progress. Preclinical testing of novel therapies in murine models As discussed above, immune activation pathways involved in initiation of SLE may not always be the same as those that are pathogenic during the effector phase. Furthermore, inflammatory environments can alter signalling cascades or provide redundant survival signals. Because the clinical effects of new therapeutics may not always be predictable from in vitro studies or studies in non-autoimmune mice, it is important to test these drugs in preclinical models. Many different mouse models of SLE now exist (Refs 3, 4, 5) and several of these have been extensively characterised and used for therapeutic studies Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 15 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine Table 2. Therapeutic trials of novel agents for systemic lupus erythematosus Drug Mechanism No. of patients Result Status Refs Anti-CD40 ligand Prevents costimulation of B cells and dendritic cells by activated T cells 85 Phase II randomised and placebo-controlled study of IDEC-131 for active SLE; improvements in disease activity scores were not achieved Open-label study of BG9588; not powered to assess clinical efficacy but clinical improvements were reported Clinical trials stopped 94 Clinical trials stopped 95 18 Abetimus sodium Unclear; crosslinks Bcell receptors and clears antiDNA antibodies from the serum 213, 317 Two time-to-renal-flare randomised and placebocontrolled studies; did not meet primary endpoints; decreased flares in a subset of patients upon post-hoc analysis Further Phase III trials in progress 142, 203 DNase Decreases antigenic load; may alter immune complexes 17 Phase I study showed no clinical effect Not in current use 204 Anti-CD22 (Eprutuzamab) Modest naive Bcell depletion; possible B-cell antiproliferative effect 14 Not powered to assess clinical efficacy; improvements in BILAG scores reported On hold 139 Anti-CD20 (Rituximab) B-cell depletion of multiple subsets; spares plasma cells .100 Several uncontrolled case series usually in combination with other agents; many reports of clinical efficacy Phase III trial in progress 126, 133, 205, 206 Anti-IL-10 Cytokine antagonist 6 Open-label pilot study of a murine antibody; not powered to assess clinical efficacy but sustained clinical improvements were reported Phase I trial in progress 187 Anti-TNF (Infliximab) Cytokine antagonist 7 Case series reporting use of infliximab in active SLE; not powered to assess clinical efficacy but clinical improvements were reported Phase II trial of etanercept in progress 167 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ (continued on next page) 16 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine Table 2. Therapeutic trials of novel agents for systemic lupus erythematosus (continued) Drug Mechanism No. of patients Result Status Refs Anti-BLySa (Belimumab) Blocks B-cell survival molecule BLyS (BAFF); B-cell depletion; spares plasma cells 449 Randomised placebocontrolled trial for active SLE; did not meet primary endpoints at 24 weeks but long-term follow up with post-hoc analysis of serologically active patients showed significantly greater improvement in treated patients than placebo Phase III trials in progress 117 a Published in abstract form only but included here because it is a large Phase III study. Abbreviations: BAFF, B-cell-activating factor belonging to the TNF family (also known as BLyS, B lymphocyte activator); BILAG, British Isles lupus assessment; SLE, systemic lupus erythematosus; TNF, tumour necrosis factor. (Ref. 200) (Table 1). Most have been used for studies of prevention or treatment of SLE nephritis or of the effects of treatment on autoantibody production and immune cell activation. In addition, the MRL/lpr mouse can be used for study of vasculitis, arthritis and skin disease, whereas the NZW/BXSB mouse can be used to study the effects of therapy on antiphospholipid syndrome (Ref. 201). It is important to recognise that murine SLE models have a number of limitations. First, mouse immune pathways are not always the same as human pathways. Second, not all clinical SLE manifestations are present in each mouse strain. In particular, there are no good mouse models for the treatment of the central nervous system manifestations of SLE. Third, responses to some therapies are strain dependent and therapies that improve disease in one model may worsen it in another. In particular, a number of differences have been observed in responses to therapy of MRL/lpr mice and NZB/W mice. Fourth, most mouse models do not undergo spontaneous disease remissions, although it is now possible to study disease relapses after remissions have been induced with biological therapies. Nevertheless, animal models allow analysis of basic immune mechanisms of disease and therapy that are not possible in human studies and can help direct appropriate clinical use of new therapeutics. Clinical trials in human SLE New agents for which the results of clinical trials have been published are shown in Table 2. Most of these trials were Phase II studies and had insufficient statistical power to assess clinical efficacy. However, several large Phase III studies are now in progress and results can be expected in the next few years. The design of SLE clinical trials will be of crucial importance in determining optimal therapies for induction of disease remission and for maintenance of disease remission. Much thought is being given to recruitment of patient populations, disease activity measurements, disease response measurements, primary outcome measures and mechanistic analyses of immunological responses in treated patients. Most clinical trials now employ the BILAG, a scoring system that measures the severity of disease in individual organ systems. Some drugs are being tested in remission induction studies whereas others are being tested in ‘time-to-flare’ studies. The choice of design will depend on the expected mechanism of action of each drug. For example, blockade of inflammatory cytokines or depletion of whole cell populations would be expected to induce remission of active disease whereas therapies that prevent naive cell activation or modulate B-cell selection would be expected to have a preventive rather than a remission Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 17 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine Plasmacytoid DC TLR ODN IFN-α Immune complexes Lymphoid organisation Anti-IFN-α ODN ODN Peptide-specific therapy FcR TLR Cytokines B cell Anti-CD20/CD22 Migration to target organs ICOS Anti-ICOSL BAFF APRIL Antigen presentation CD40L/CD40 CD28/B7 TLR Myeloid DC BAFF blockade CD28/B7 CTLA4Ig/anti-CD40 CD40L/CD40 T cell Interactions of the innate and acquired immune system amplify immune responses in systemic lupus erythematosus Expert Reviews in Molecular Medicine © 2008 Cambridge University Press Figure 5. Interactions of the innate and acquired immune system amplify immune responses in systemic lupus erythematosus. Therapeutic interventions for SLE (shown in red) target multiple arms of the immune system (see Ref. 143 for review). Abbreviations: APRIL, A proliferation inducing ligand; BAFF, B-cellactivating factor belonging to the TNF family; DC, dendritic cell; ICOS, inducible costimulator; IFN-a, interferon a; ODN, oligodeoxynucleotide; TLR, Toll-like receptor. induction effect. Testing in preclinical models may help direct trial design for each new agent. The range of new drugs being developed reflects the global nature of the immune defects in SLE (Fig. 5). Approaches include measures to decrease antigen load (DNase), measures to dampen the innate immune system (ODN and IFN-a blockade), drugs that antagonise activation of the acquired immune system (costimulatory blockade, modulation of BCR signals), blockade of the soluble mediators derived from the effector arm of the immune response (cytokine antagonists), protection of target organs from damage (chemokine inhibitors, complement inhibitors) and even depletion of whole cell populations (B-cell depletion, stem cell transplant). In addition, there are agents designed to specifically target autoreactive B cells through induction of antiidiotypic antibodies. The scope of this article does not allow discussion of all the new drugs being developed, but for a comprehensive review see Ref. 143. The challenge will now be to determine at which stage of disease each reagent is most likely to be effective and which reagents will work together. Optimal therapy will also require continued research that will eventually allow identification of the underlying immune defect in each patient. The ultimate goal is to selectively correct deficits that contribute to autoimmunity without impacting protective immunity. Acknowledgements and funding Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ M.R. and A.D. are supported by grants from the NY SLE Foundation, NY Arthritis Foundation, Lupus Research Institute, Alliance for Lupus Research and the NIH. M.R. is an Arthritis Foundation Young Scholar. A.D. is a Kirkland Lupus Scholar. We thank our panel of peer reviewers for helpful suggestions in improving this manuscript. 18 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press References 1 Wardemann, H. et al. (2003) Predominant autoantibody production by early human B cell precursors. Science 301, 1374-1377 2 Arbuckle, M.R. et al. (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349, 1526-1533 3 Fairhurst, A.M., Wandstrat, A.E. and Wakeland, E.K. (2006) Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol 92, 1-69 4 Theofilopoulos, A.N. and Kono, D.H. (1999) The genes of systemic autoimmunity. Proc Assoc Am Physicians 111, 228-240 5 Lauwerys, B.R. and Wakeland, E.K. (2005) Genetics of lupus nephritis. Lupus 14, 2-12 6 Grimaldi, C.M. et al. (2002) Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest 109, 1625-1633 7 Cornall, R.J. and Goodnow, C.C. (1998) B cell antigen receptor signalling in the balance of tolerance and immunity. Novartis Found Symp 215, 21-40 8 Mahoney, J.A. and Rosen, A. (2005) Apoptosis and autoimmunity. Curr Opin Immunol 17, 583-588 9 Navratil, J.S., Liu, C.C. and Ahearn, J.M. (2006) Apoptosis and autoimmunity. Immunol Res 36, 3-12 10 Bickerstaff, M.C. et al. (1999) Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med 5, 694-697 11 Manson, J.J., Mauri, C. and Ehrenstein, M.R. (2005) Natural serum IgM maintains immunological homeostasis and prevents autoimmunity. Springer Semin Immunopathol 26, 425-432 12 Manderson, A.P., Botto, M. and Walport, M.J. (2004) The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22, 431-456 13 Lee-Kirsch, M.A. et al. (2007) Mutations in the gene encoding the 30 -50 DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet 39, 1065-1067 14 Christensen, S.R. and Shlomchik, M.J. (2007) Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol 19, 11-23 15 Lenert, P.S. (2006) Targeting Toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus. Arthritis Res Ther 8, 203 expert reviews in molecular medicine 16 Wagner, H. and Bauer, S. (2006) All is not Toll: new pathways in DNA recognition. J Exp Med 203, 265-268 17 Lau, C.M. et al. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 202, 1171-1177 18 Wagner, H. (2006) Endogenous TLR ligands and autoimmunity. Adv Immunol 91, 159-173 19 Rutz, M. et al. (2004) Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pHdependent manner. Eur J Immunol 34, 2541-2550 20 Akira, S., Uematsu, S. and Takeuchi, O. (2006) Pathogen recognition and innate immunity. Cell 124, 783-801 21 Honda, K., Takaoka, A. and Taniguchi, T. (2006) Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349-360 22 Pandey, S. and Agrawal, D.K. (2006) Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol 84, 333-341 23 Sigurdsson, S. et al. (2005) Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet 76, 528-537 24 Ronnblom, L. and Alm, G.V. (2003) Systemic lupus erythematosus and the type I interferon system. Arthritis Res Ther 5, 68-75 25 Mathian, A. et al. (2005) IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol 174, 2499-2506 26 Pasare, C. and Medzhitov, R. (2003) Toll pathwaydependent blockade of CD4þ CD25þ T cellmediated suppression by dendritic cells. Science 299, 1033-1036 27 Krutzik, S.R. et al. (2005) TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 11, 653-660 28 Logue, E.C. et al. (2006) ICOS-induced B7 h shedding on B cells is inhibited by TLR7/8 and TLR9. J Immunol 177, 2356-2364 29 Ehlers, M. and Ravetch, J.V. (2007) Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol 28, 74-79 30 Tian, J. et al. (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8, 487-496 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 19 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press 31 Christensen, S.R. et al. (2006) Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417-428 32 Ehlers, M. et al. (2006) TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med 203, 553-561 33 Wu, X. and Peng, S.L. (2006) Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum 54, 336-342 34 Berland, R. et al. (2006) Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 25, 429-440 35 Pisitkun, P. et al. (2006) Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669-1672 36 Akkerman, A. et al. (2004) CTLA4Ig prevents initiation but not evolution of anti-phospholipid syndrome in NZW/BXSB mice. Autoimmunity 37, 445-451 37 Kanzler, H. et al. (2007) Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med 13, 552-559 38 Dong, L. et al. (2005) Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupusprone NZB x NZW mice. Arthritis Rheum 52, 651-658 39 Anders, H.J. and Schlondorff, D. (2007) Toll-like receptors: emerging concepts in kidney disease. Curr Opin Nephrol Hypertens 16, 177-183 40 Nimmerjahn, F. and Ravetch, J.V. (2006) Fcgamma receptors: old friends and new family members. Immunity 24, 19-28 41 McGaha, T.L., Sorrentino, B. and Ravetch, J.V. (2005) Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science 307, 590-593 42 Clatworthy, M.R. et al. (2007) Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A 104, 7169-7174 43 Mackay, M. et al. (2006) Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med 203, 2157-2164 44 Su, K. et al. (2007) Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol 178, 3272-3280 expert reviews in molecular medicine 45 Bolland, S. and Ravetch, J.V. (2000) Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity 13, 277-285 46 Fukuyama, H., Nimmerjahn, F. and Ravetch, J.V. (2005) The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin Gþ anti-DNA plasma cells. Nat Immunol 6, 99-106 47 Harley, J.B., Kelly, J.A. and Kaufman, K.M. (2006) Unraveling the genetics of systemic lupus erythematosus. Springer Semin Immunopathol 28, 119-130 48 Karassa, F.B., Trikalinos, T.A. and Ioannidis, J.P. (2004) The role of FcgammaRIIA and IIIA polymorphisms in autoimmune diseases. Biomed Pharmacother 58, 286-291 49 Nimmerjahn, F. and Ravetch, J.V. (2007) The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med 204, 11-15 50 Diamond, B. et al. (1992) The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol 10, 731-757 51 Abbas, A.K. et al. (2004) T cell tolerance and autoimmunity. Autoimmun Rev 3, 471-475 52 Miyara, M. and Sakaguchi, S. (2007) Natural regulatory T cells: mechanisms of suppression. Trends Mol Med 13, 108-116 53 Mondino, A. and Mueller, D.L. (2007) mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol 19, 162-172 54 Vincenti, F. and Luggen, M. (2007) T cell costimulation: a rational target in the therapeutic armamentarium for autoimmune diseases and transplantation. Annu Rev Med 58, 347-358 55 Lohr, J. et al. (2003) The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol 4, 664-669 56 Mellor, A.L. et al. (2003) Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol 171, 1652-1655 57 Mellor, A. (2005) Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun 338, 20-24 58 Ueda, H. et al. (2003) Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423, 506-511 59 Mirenda, V. et al. (2007) Physiologic and aberrant regulation of memory T-cell trafficking by the Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 20 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press 60 61 62 63 64 65 66 67 68 69 70 71 72 costimulatory molecule CD28. Blood 109, 2968-2977 Davidson, A. et al. (2005) Block and tackle: CTLA4Ig takes on lupus. Lupus 14, 197-203 Moreland, L.W. et al. (2002) Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebocontrolled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum 46, 1470-1479 Finck, B.K., Linsley, P.S. and Wofsy, D. (1994) Treatment of murine lupus with CTLA4Ig. Science 265, 1225-1227 Daikh, D.I. et al. (1997) Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol 159, 3104-3108 Wang, X. et al. (2002) Mechanism of action of combined short-term CTLA4Ig and anti-CD40 ligand in murine systemic lupus erythematosus. J Immunol 168, 2046-2053 Schiffer, L. et al. (2003) Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol 171, 489-497 Greenwald, R.J., Latchman, Y.E. and Sharpe, A.H. (2002) Negative co-receptors on lymphocytes. Curr Opin Immunol 14, 391-396 Hutloff, A. et al. (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263-266 Lohning, M. et al. (2003) Expression of ICOS in vivo defines CD4þ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med 197, 181-193 Rasheed, A.U. et al. (2006) Follicular B helper Tcell activity is confined to CXCR5(hi)ICOS(hi) CD4 Tcells and is independent of CD57 expression. Eur J Immunol 36, 1892-1903 McAdam, A.J. et al. (2001) ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102-105 Dong, C. et al. (2001) ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409, 97-101 Shilling, R.A., Bandukwala, H.S. and Sperling, A.I. (2006) Regulation of T:B cell interactions by the inducible costimulator molecule: does ICOS “induce” disease? Clin Immunol 121, 13-18 expert reviews in molecular medicine 73 Hutloff, A. et al. (2004) Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum 50, 3211-3220 74 Vinuesa, C.G. et al. (2005) A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452-458 75 Iwai, H. et al. (2003) Involvement of inducible costimulator-B7 homologous protein costimulatory pathway in murine lupus nephritis. J Immunol 171, 2848-2854 76 Sharpe, A.H. et al. (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8, 239-245 77 Ansari, M.J. et al. (2003) The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198, 63-69 78 Fife, B.T. et al. (2006) Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med 203, 2737-2747 79 Okazaki, T., Iwai, Y. and Honjo, T. (2002) New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol 14, 779-782 80 Prokunina, L. et al. (2002) A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet 32, 666-669 81 Watanabe, N. et al. (2003) BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4, 670-679 82 Sedy, J.R. et al. (2005) B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6, 90-98 83 Greenwald, R.J., Freeman, G.J. and Sharpe, A.H. (2005) The B7 family revisited. Annu Rev Immunol 23, 515-548 84 Foell, J. et al. (2003) CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest 111, 1505-1518 85 Kim, J. et al. (2005) Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4þ T cells. Blood 105, 2206-2213 86 Choi, B.K. et al. (2004) 4-1BB-dependent inhibition of immunosuppression by activated CD4þ CD25þ T cells. J Leukoc Biol 75, 785-791 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 21 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews 87 Sun, Y. et al. (2002) Costimulatory moleculetargeted antibody therapy of a spontaneous autoimmune disease. Nat Med 8, 1405-1413 88 Salomon, B. and Bluestone, J.A. (2001) Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 19, 225-252 89 Castigli, E. et al. (1994) CD40-deficient mice generated by recombination-activating gene-2deficient blastocyst complementation. Proc Natl Acad Sci U S A 91, 12135-12139 90 Grewal, I.S. and Flavell, R.A. (1998) CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16, 111-135 91 Vogel, L.A. and Noelle, R.J. (1998) CD40 and its crucial role as a member of the TNFR family. Semin Immunol 10, 435-442 92 Wang, X. et al. (2003) Effects of anti-CD154 treatment on B cells in murine systemic lupus erythematosus. Arthritis Rheum 48, 495-506 93 Sidiropoulos, P.I. and Boumpas, D.T. (2004) Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 13, 391-397 94 Kalunian, K.C. et al. (2002) Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 46, 3251-3258 95 Boumpas, D.T. et al. (2003) A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 48, 719-727 96 Huang, W. et al. (2002) The effect of anti-CD40 ligand antibody on B cells in human SLE. Arthritis Rheum 46, 1554-1562 97 Grammer, A.C. et al. (2001) Normalization of peripheral B cells following treatment of active SLE patients with humanized anti-CD154 MAb (5c8, BG9588) (Abstract). Arthritis Rheum 44, S282 98 Mohan, C. et al. (1995) Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol 154, 1470-1480 99 Drachman, J.G. et al. (2005) A humanized antiCD40 monoclonal antibody (SGN-40) demonstrates antitumor activity in non-Hodgkin’s lymphoma: initiation of a Phase I clinical trial. J Clin Oncol 23, 6572 100 Sutherland, A.P., Mackay, F. and Mackay, C.R. (2006) Targeting BAFF: immunomodulation for in molecular medicine 101 102 103 104 105 106 107 108 109 110 111 112 113 114 autoimmune diseases and lymphomas. Pharmacol Ther 112, 774-786 Ramanujam, M. and Davidson, A. (2004) The current status of targeting BAFF/BLyS for autoimmune diseases. Arthritis Res Ther 6, 197-202 Mackay, F. et al. (2003) BAFFAND APRIL: a tutorial on B cell survival. Annu Rev Immunol 21, 231-264 O’Connor, B.P. et al. (2004) BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199, 91-98 Ingold, K. et al. (2005) Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med 201, 1375-1383 Schiemann, B. et al. (2001) An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293, 2111-2114 Ramanujam, M. et al. (2006) Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest 116, 724-734 Vora, K.A. et al. (2003) Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol 171, 547-551 Jacob, C.O. et al. (2006) Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol 177, 2671-2680 Groom, J.R. et al. (2007) BAFF and MyD88 signals promote a lupuslike disease independent of Tcells. J Exp Med 204, 1959-1971 Lesley, R. et al. (2004) Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20, 441-453 Thien, M. et al. (2004) Excess BAFF rescues selfreactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785-798 Martin, F. and Chan, A.C. (2006) B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol 24, 467-496 Zhang, J. et al. (2001) Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 166, 6-10 Cheema, G.S. et al. (2001) Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 44, 1313-1319 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 22 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press 115 Chang, S.K. et al. (2006) A role for BLyS in the activation of innate immune cells. Blood 108, 2687-2694 116 Ramanujam, M. et al. (2004) Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol 173, 3524-3534 117 Ginzler, E. et al. (2007) Novel combined response endpoint shows that belimumab (fully human monoclonal antibody to B-lymphocyte stimulator [BLyS]) improves or stabilizes SLE disease activity in a phase 2 trial. Presented at EULAR 2007 Meeting (13 –16 June 2007; Barcelona, Spain), Abstract OP0018, http://abstract.mci-group.com/ cgi-bin/mc/printabs.pl?APP=EULAR2007SCIEabstract&TEMPLATE=&keyf=0267&showHide =show&client= 118 Grammer, A.C. and Lipsky, P.E. (2003) B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther 5, S22-S27 119 Riley, J.K. and Sliwkowski, M.X. (2000) CD20: a gene in search of a function. Semin Oncol 27, 17-24 120 Uchida, J. et al. (2004) Mouse CD20 expression and function. Int Immunol 16, 119-129 121 Clynes, R.A. et al. (2000) Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 6, 443-446 122 Uchida, J. et al. (2004) The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 199, 1659-1669 123 Gong, Q. et al. (2005) Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174, 817-826 124 Hamaguchi, Y. et al. (2005) The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol 174, 4389-4399 125 Lin, W.Y. et al. (2007) Anti-BR3 antibodies - a new class of B cell immunotherapy combining cellular depletion and survival blockade. Blood 110, 3959-3967 126 Sfikakis, P.P., Boletis, J.N. and Tsokos, G.C. (2005) Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol 17, 550-557 127 Looney, R.J. et al. (2004) B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50, 2580-2589 expert reviews in molecular medicine 128 Leandro, M.J. et al. (2002) An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 46, 2673-2677 129 van Vollenhoven, R.F. et al. (2004) Biopsy-verified response of severe lupus nephritis to treatment with rituximab (anti-CD20 monoclonal antibody) plus cyclophosphamide after biopsy-documented failure to respond to cyclophosphamide alone. Scand J Rheumatol 33, 423-427 130 Anolik, J.H. et al. (2003) The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 48, 455-459 131 Anolik, J.H. et al. (2004) Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 50, 3580-3590 132 Leandro, M.J. et al. (2006) Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54, 613-620 133 Ng, K.P. et al. (2007) B cell depletion therapy in systemic lupus erythematosus: long-term followup and predictors of response. Ann Rheum Dis 66, 1259-1262 134 Tedder, T.F. et al. (1997) CD22, a B lymphocytespecific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol 15, 481-504 135 Crocker, P.R., Paulson, J.C. and Varki, A. (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7, 255-266 136 Otipoby, K.L. et al. (1996) CD22 regulates thymusindependent responses and the lifespan of B cells. Nature 384, 634-637 137 O’Keefe, T.L. et al. (1999) Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med 189, 1307-1313 138 Leonard, J.P. et al. (2004) Epratuzumab, a humanized anti-CD22 antibody, in aggressive nonHodgkin’s lymphoma: phase I/II clinical trial results. Clin Cancer Res 10, 5327-5334 139 Dorner, T. et al. (2006) Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther 8, R74 140 Jacobi, A.M. et al. (2007) Differential effects of epratuzumab on peripheral blood B cells of SLE patients versus normal controls. Ann Rheum Dis [doi:10.1136/ard.2007.075762] 141 Carnahan, J. et al. (2007) Epratuzumab, a CD22targeting recombinant humanized antibody with a Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 23 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews 142 143 144 145 146 147 148 149 150 151 152 153 different mode of action from rituximab. Mol Immunol 44, 1331-1341 Alarcon-Segovia, D. et al. (2003) LJP 394 for the prevention of renal flare in patients with systemic lupus erythematosus: results from a randomized, double-blind, placebo-controlled study. Arthritis Rheum 48, 442-454 Wiesendanger, M. et al. (2006) Novel therapeutics for systemic lupus erythematosus. Curr Opin Rheumatol 18, 227-235 Herrero, C. et al. (2003) Reprogramming of IL-10 activity and signaling by IFN-gamma. J Immunol 171, 5034-5041 Hu, X. et al. (2007) Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol 82, 237-243 Liang, B. et al. (2006) Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology 119, 296-305 Kishimoto, T. (2005) Interleukin-6: from basic science to medicine – 40 years in immunology. Annu Rev Immunol 23, 1-21 Mihara, M., Nishimoto, N. and Ohsugi, Y. (2005) The therapy of autoimmune diseases by antiinterleukin-6 receptor antibody. Expert Opin Biol Ther 5, 683-690 Linker-Israeli, M. et al. (1991) Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol 147, 117-123 Tackey, E., Lipsky, P.E. and Illei, G.G. (2004) Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus 13, 339-343 Mihara, M. et al. (1998) IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol 112, 397-402 Finck, B.K., Chan, B. and Wofsy, D. (1994) Interleukin 6 promotes murine lupus in NZB/ NZW F1 mice. J Clin Invest 94, 585-591 Illei, G.G. et al. (2006) Tocilizumab (humanized anti IL-6 receptor monoclonal antibody) in patients with systemic lupus erythematosus (SLE): safety, tolerability and preliminary efficacy. Presented at American College of Rheumatology Meeting, (10–15 November 2006; Washington DC, USA). Abstract no. 500, http:// www.abstractsonline.com/viewer/ viewAbstractPrintFriendly.asp?CKey={6F07625DEB8C-4B2A-9416-747B62375600}&SKey= {9EABBCE4-6F21-4386-9659-6C0982180B4A}& MKey={C297FAF7-2B4C-45F5-A662- in molecular medicine 154 155 156 157 158 159 160 161 162 163 164 165 166 167 0972E559ED7D}&AKey={AA45DD66-F1134CDD-8E62-01A05F613C0D} Hill, C.M. and Lunec, J. (1996) The TNF-ligand and receptor superfamilies: controllers of immunity and the Trojan horses of autoimmune disease? Mol Aspects Med 17, 455-509 Jacob, C.O. and McDevitt, H.O. (1988) Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature 331, 356-358 Kontoyiannis, D. and Kollias, G. (2000) Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol 30, 2038-2047 Mountz, J.D. (2001) Re: collagen-induced arthritis in TNF receptor-1-deficient mice: TNF receptor-2 can modulate arthritis in the absence of TNF receptor 1. Clin Immunol 99, 305-307 Romas, E., Gillespie, M.T. and Martin, T.J. (2002) Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone 30, 340-346 Feldmann, M. and Maini, R.N. (2001) Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 19, 163-196 Sandborn, W.J. and Hanauer, S.B. (1999) Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis 5, 119-133 Feldmann, M. and Maini, R.N. (2003) Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 9, 1245-1250 Tobin, A.M. and Kirby, B. (2005) TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs 19, 47-57 Kollias, G. (2005) TNF pathophysiology in murine models of chronic inflammation and autoimmunity. Semin Arthritis Rheum 34, 3-6 Swale, V.J. et al. (2003) Etanercept-induced systemic lupus erythematosus. Clin Exp Dermatol 28, 604-607 Tipping, P.G. and Holdsworth, S.R. (2007) Cytokines in glomerulonephritis. Semin Nephrol 27, 275-285 Aringer, M. et al. (2004) Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum 50, 3161-3169 Aringer, M. et al. (2007) Effects of short-term infliximab therapy on autoantibodies in systemic Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 24 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews 168 169 170 171 172 173 174 175 176 177 178 179 180 lupus erythematosus. Arthritis Rheum 56, 274-279 Valencia, X. et al. (2007) Deficient CD4þ CD25 high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 178, 2579-2588 Chan, F.K. and Lenardo, M.J. (2002) Tumor Necrosis Factor Family Ligands and Receptors in the Immune System: Targets for Future Pharmaceuticals. Drug News Perspect 15, 483-490 Locksley, R.M., Killeen, N. and Lenardo, M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487-501 Fu, Y.X. and Chaplin, D.D. (1999) Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 17, 399-433 Kassiotis, G. and Kollias, G. (2001) Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med 193, 427-434 Pascual, V., Farkas, L. and Banchereau, J. (2006) Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol 18, 676-682 Ronnblom, L. and Alm, G.V. (2002) The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum Immunol 63, 1181-1193 Bennett, L. et al. (2003) Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 197, 711-723 Baechler, E.C. et al. (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100, 2610-2615 Koutouzov, S., Mathian, A. and Dalloul, A. (2006) Type-I interferons and systemic lupus erythematosus. Autoimmun Rev 5, 554-562 Meyers, J.A. et al. (2006) Blockade of TLR9 agonistinduced type I interferons promotes inflammatory cytokine IFN-gamma and IL-17 secretion by activated human PBMC. Cytokine 35, 235-246 Nagai, T. et al. (2003) Timing of IFN-beta exposure during human dendritic cell maturation and naive Th cell stimulation has contrasting effects on Th1 subset generation: a role for IFN-beta-mediated regulation of IL-12 family cytokines and IL-18 in naive Th cell differentiation. J Immunol 171, 5233-5243 Dhodapkar, K.M. et al. (2007) Selective blockade of the inhibitory Fc gamma receptor (Fc gamma RIIB) in human dendritic cells and monocytes induces a in molecular medicine 181 182 183 184 185 186 187 188 189 190 191 192 193 type I interferon response program. J Exp Med 204, 1359-1369 Park, Y.B. et al. (1998) Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 16, 283-288 Hagiwara, E. et al. (1996) Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10: interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum 39, 379-385 Ji, J.D. et al. (2003) Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med 197, 1573-1583 Ishida, H. et al. (1994) Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med 179, 305-310 Blenman, K.R. et al. (2006) IL-10 regulation of lupus in the NZM2410 murine model. Lab Invest 86, 1136-1148 Yin, Z. et al. (2002) IL-10 regulates murine lupus. J Immunol 169, 2148-2155 Llorente, L. et al. (2000) Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum 43, 1790-1800 Jacob, C.O., van der Meide, P.H. and McDevitt, H.O. (1987) In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med 166, 798-803 Ozmen, L. et al. (1995) Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferongamma receptor inhibits the onset of glomerulonephritis. Eur J Immunol 25, 6-12 Balomenos, D., Rumold, R. and Theofilopoulos, A.N. (1998) Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest 101, 364-371 Singh, R.R. et al. (2003) Differential contribution of IL-4 and STAT6 vs STAT4 to the development of lupus nephritis. J Immunol 170, 4818-4825 Weaver, C.T. et al. (2007) IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol 25, 821-852 Clynes, R., Dumitru, C. and Ravetch, J.V. (1998) Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052-1054 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 25 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press 194 Bergtold, A. et al. (2006) FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol 177, 7287-7295 195 Chan, O.T. et al. (1999) A novel mouse with B cells but lacking serum antibody reveals an antibodyindependent role for B cells in murine lupus. J Exp Med 189, 1639-1648 196 Matsumoto, K. et al. (2003) Fc receptorindependent development of autoimmune glomerulonephritis in lupus-prone MRL/lpr mice. Arthritis Rheum 48, 486-494 197 Peterson, K.S. et al. (2004) Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest 113, 1722-1733 198 Anders, H.J. et al. (2004) Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRLFas(lpr) mice. J Am Soc Nephrol 15, 1504-1513 199 Anders, H.J., Ninichuk, V. and Schlondorff, D. (2006) Progression of kidney disease: blocking leukocyte recruitment with chemokine receptor CCR1 antagonists. Kidney Int 69, 29-32 200 Hahn, B.H. (1997) An overview of the pathogenesis of systemic lupus erythematosus. In Dubois’s Lupus Erythematosus (5th edn) (Wallace D.J. and Hahn B.H., eds), pp 69-76, Williams & Wilkins expert reviews in molecular medicine 201 Akkerman, A. et al. (2004) CTLA4Ig prevents initiation but not evolution of anti-phospholipid syndrome in NZW/BXSB mice. Autoimmunity 37, 445-451 202 Theofilopoulos, A.N. and Dixon, F.J. (1985) Murine models of systemic lupus erythematosus. Adv Immunol 37, 269-390 203 Furie, R. (2006) Abetimus sodium (riquent) for the prevention of nephritic flares in patients with systemic lupus erythematosus. Rheum Dis Clin North Am 32, 149-156 204 Davis, J.C., Jr. et al. (1999) Recombinant human DNase I (rhDNase) in patients with lupus nephritis. Lupus 8, 68-76 205 Gunnarsson, I. et al. (2007) Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum 56, 1263-1272 206 Eisenberg, R. (2006) Targeting B cells in SLE: the experience with rituximab treatment (anti-CD20). Endocr Metab Immune Disord Drug Targets 6, 345-350 207 Schiffer, L. et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol (in press) 208 Kilmon, M. et al. (2005) Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol 175, 37-41 Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ 26 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press expert reviews in molecular medicine Further reading, resources and contacts Publications Wiesendanger, M. et al. (2006) Novel therapeutics for systemic lupus erythematosus. Curr Opin Rheumatol 18, 227-235 This review paper provides a comprehensive summary of new therapeutics being developed for SLE and the rationale for their use. Furie, R. (2006) Abetimus sodium (riquent) for the prevention of nephritic flares in patients with systemic lupus erythematosus. Rheum Dis Clin North Am 32, 149-156 This paper provides a balanced overview of the entire clinical experience with the DNA analogue abetimus sodium for the treatment of SLE. Fairhurst, A.M., Wandstrat, A.E. and Wakeland, E.K. (2006) Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol 92, 1-69 A comprehensive review of the genetics of SLE. Davidson, A. and Aranow, C. (2006) Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol 18, 468-475 An up-to-date review of pathogenesis and current therapeutic approaches for SLE nephritis. Websites A list of current clinical trials in SLE can be found at: http://www.clinicaltrials.gov/ct/search; jsessionid¼7FB635BE3203D66B6A49A25BD493C13B?term ¼SLE The official site of the Lupus Research Institute and the NY Lupus Foundation: http://www.lupusny.org/ An NIH summary statement on SLE can be found at: http://www.niams.nih.gov/hi/topics/lupus/slehandout/index.htm Features associated with this article Figures Figure 1. The innate immune system in systemic lupus erythematosus. Figure 2. Members of the CD28 –B7 family mediate both costimulation and coinhibition. Figure 3. The events that lead to renal failure in systemic lupus erythematosus. Figure 4. Therapies for systemic lupus erythematosus depend on the stage of disease. Figure 5. Interactions of the innate and acquired immune system amplify immune responses in systemic lupus erythematosus. Tables Table 1. Common mouse models of systemic lupus erythematosus used for therapeutic studies. Table 2. Therapeutic trials of novel agents for systemic lupus erythematosus. Targeting of the immune system in systemic lupus erythematosus http://www.expertreviews.org/ Citation details for this article Meera Ramanujam and Anne Davidson (2008) Targeting of the immune system in systemic lupus erythematosus. Expert Rev. Mol. Med. Vol. 10, e2, January 2008, doi:10.1017/S1462399408000562 27 Accession information: doi:10.1017/S1462399408000562; Vol. 10; e2; January 2008 & 2008 Cambridge University Press