* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Proteins - UF Macromolecular Structure Group
Fatty acid metabolism wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Paracrine signalling wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Magnesium transporter wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Catalytic triad wikipedia , lookup
Interactome wikipedia , lookup
Signal transduction wikipedia , lookup
Point mutation wikipedia , lookup
Western blot wikipedia , lookup
Peptide synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Biosynthesis wikipedia , lookup
Page 8/6: The cell
Where to start: Proteins (control a cell) (start/end products)
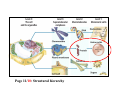
Page 11/10: Structural hierarchy
Proteins
Phenotype of organism
3 Dimensional structure
Function by interaction
THE PROTEIN DATA BANK
http://www.rcsb.org/pdb/
Two-dimensional gel showing more than 1,000 different
proteins from E. Coli - Page 95/91
The function of proteins depend on their
ability to interact with other molecules
Proteome:
Entire complement of an organisms proteins:
yeast ≈ 6,000 proteins
human ≈ 32,000 proteins
Proteins can bind to:
Substrate Molecules (small molecules)
Cell Receptors
Nucleic Acids (DNA/RNA)
Polysaccharides
Lipids
The environment in the cell is crowded
Protein interactions are
highly specific
(avoid non-productive
interactions)
Molecule that interacts with
protein is called the
LIGAND (or substrate)
This can be another protein
Examples of Functions
Structure
Transport
Storage
Catalysis
Receptors
Antibodies
Movement (contraction and motility)
Amino Acids (building block of proteins)
(Amino group)
NH2
(Carboxyl group)
COOH
(alpha carbon)
(side chain)
Ampholytes molecules that contain both acidic and basic groups
- and will exist mostly as zwitterions in a certain range of pH
Side Chains R
R = H The simplest amino acid Glycine
20 “common” amino acids
Differ in properties because of R
Many ways to classify amino acids types
based on properties
Hydrophobic-aliphatic
Non-polar methyl- or methylene- groups
Usually located on the interior of the protein
All of these side chains except for alanine are bifurcated
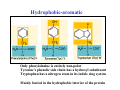
Hydrophobic-aromatic
Only phenylalanine is entirely non-polar
Tyrosine's phenolic side chain has a hydroxyl substituent
Tryptophan has a nitrogen atom in its indole ring system
Mainly buried in the hydrophobic interior of the protein
Neutral-polar
Contain small aliphatic side chains containing polar groups
Cannot readily ionize
Acidic
Have carboxyl side chains and are therefore negatively charged
at physiological pH (around ~ pH 7)
The strongly polar nature of these residues means that they are most
often found on the surface of proteins
Basic
Of the basic amino acid side chains, histidine has the lowest pKa
(around 6)
Lysine and arginine are more strongly basic and are positively
charged at physiological pH's
Conformationally important
Glycine and proline are unique as they influence the conformation
of the polypeptide chain.
Glycine essentially lacks a side chain and therefore can adopt
conformations which are sterically forbidden for other amino acids.
Proline is the most rigid amino acids since its side chain is covalently
linked with the main chain nitrogen.
Page 78/73
One and Three letter codes
Alanine
Cysteine
Aspartate
Glutamate
Phenylalanine
Glycine
Histidine
Isoleucine
Lysine
Leucine
Methionine
Asparagine
Proline
Glutamine
Arginine
Serine
Threonine
Valine
Tryptophan
Tyrosine
Ala
Cys
Asp
Glu
Phe
Gly
His
Ile
Lys
Leu
Met
Asn
Pro
Gln
Arg
Ser
Thr
Val
Trp
Tyr
A
C
D
E
F
G
H
I
K
L
M
N
P
Q
R
S
T
V
W
Y
first amino acid (AA) alphabetically
only AA to start with C
asparDate (or if you prefer, asparDic acid)
gluEtamate (or gluEtamic acid)
Fenylalanine
alphabetically first of the AAs starting with G
only AA starting with H
only AA starting with I
(I don't have a good mnemonic for this one)
alphabetically first of the AAs starting with L
only AA starting with M
asparagiNe
starts with P and is P-shaped (imino acid ring)
Q-tamine
R-ginine
only AA starting with S
alphabetically first AA starting with T
only AA starting with V
twyptophan (Elmer Fudd's favorite amino acid)
tYrosine
Summary
AMINO ACIDS
CODE
THREE
ONE LETTER
Alanine
Arginine
Ala
Arg
A
R
Asparagine
Asn
N
Aspartic acid Asp
D
Cysteine
Cys
C
Glutamic acid Glu
E
Glutamine
Glycine
Gln
Gly
Q
G
Histidine
His
H
CHARACTERISTICS
hydrophobic
free amino group makes it basic and
hydrophilic
carbohydrate can be covalently linked
("N- linked) to its -NH
free carboxyl group makes it acidic and
hydrophilic
oxidation of their sulfhydryl (-SH)
groups link 2 Cys (S-S)
free carboxyl group makes it acidic and
hydrophilic
moderately hydrophilic
so small it is amphiphilic (can exist in
any surroundings)
base/acid and hydrophilic
Isoleucine
Ile
Leucine
Leu
Lysine
Lys
Methionine Met
Phenylalanine Phe
Proline
Pro
Serine
Ser
I
L
K
M
F
P
S
Threonine
linked
Tryptophan
Tyrosine
Thr
T
Trp
Tyr
W
Y
Valine
Val
V
hydrophobic
hydrophobic
strongly basic and hydrophilic
hydrophobic
very hydrophobic
causes kinks in the chain
carbohydrate can be covalently
linked ("O-linked") to its -OH
carbohydrate can be covalently
("O-linked") to its -OH
scarce in most plant proteins
-OH group makes it moderately
hydrophilic
hydrophobic