* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Presentation part1-201210091211
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Genetic code wikipedia , lookup
Magnesium transporter wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Western blot wikipedia , lookup
Proteolysis wikipedia , lookup
Lipid signaling wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Butyric acid wikipedia , lookup
Citric acid cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Biosynthesis wikipedia , lookup
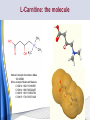
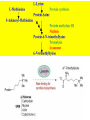
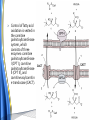
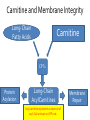
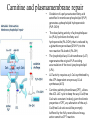
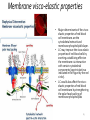
Carnitine: an overview Prof.Gianfranco Peluso M.D., Ph.D. Research Director IBP-National Research Council University of Piemonte Orientale 'A. Avogadro' 1 – Trimethylated aminoacid – Zwitterion – Sources: O CH3 + CH3 N CH3 CH2 CH OH - CH2 C O • Diet: red meats, dairies • Endogenous: protein catabolism L-Carnitine: the molecule Molecular Formula C7 H16 N O3 + Natural Isotopic Abundance Mass 162.20684 Mono-Isotopic Molecular Masses C12N14: 162.1130183851 C13N14: 169.1365022497 C12N15: 163.1100532783 C13N15: 170.1335371429 L-Carnitine and it´s Derivatives CH Free L-Carnitine + R: O C O CH3 N CH2 CH CH C 2 OCH 3 CH Acyl- L-Carnitine OH 3 + 3 O R CH3 N CH2 CH CH C 2 OCH 3 O CH3 AcetylL-Carnitine O C O (CH2)6 CH3 OctanoylL-Carnitine C (CH2)14 PalmitoylL-Carnitine CH3 L-Carnitine a natural compound of the body Some basic characteristics of distribution: a Whole body stock (70 kilogram): approx. 20 gram Portion in skeletal muscle (30 kilogram): approx. 19 gram / 95% (3,9 µmol/g) a, b Portion in heart (300 gram): 0,25 gram (4,8 µmol/g) Portion in blood (5 Liter): 0,04 gram (0,05 µmol/ml) Fraction of Free L-Carnitine (FC): about 75 - 80 % c Fraction of Acyl-L-Carnitine (AC): about 20 - 25 % c Ratio AC / FC in plasma: < 0,25 a a,b c According to: a Scholte et al., 1987 b Opalka et al., 2001 c Boulat et al., 1993 Plasma Carnitine concentration reference values Normally low Ratio AC/FC in plasma: 0,25 Concentration of Carnitine (μmol/l) in females and males and Acyl / Free ratio (mol/mol) in the reference population N Mean SD Median Value Min Max P2.5 P97.5 Total 180 38.1 7.6 38 18 57 22 52 Free 180 31.7 6.9 32 14 48 20 44 Acyl 180 6.47 3.3 6 1 18 1 14 Acyl / Free 180 0.21 1.12 0.18 0.02 Females 0.65 0.03 0.41 Males Total 160 43.6 7.3 43.5 27 60 29.5 58 Free 160 37.8 6.6 37.5 22 53 23 52 Acyl 160 5.8 4.9 5 1 21 1 13.5 Acyl / Free 160 0.16 0.1 0.15 0.02 0.03 0.56 0.54 N = Number of specimens; M = Minimal value; Maximal value; P2.5 = 2.5 percentile; P97.5 = 97.5 percentile ; SD = Standard Deviation Boulat et al., Eur J Clin Chem Clin Biochem 31(9), 1993 L-Carnitine: the molecule Although the racemic form of carnitine has been used clinically in the past, the L-form elicits the desired biochemical actions, and only the enantiomerically pure L-carnitine (levocarnitine) has a role in human medicine. The carnitine system consists of L-carnitine and acylcarnitine esters and the enzymes and transport proteins required for their metabolism and transport, including carnitine acyltransferases, mitochondrial carnitine acylcarnitine translocases, plasma membrane carnitine importers, and the carnitine biosynthesis pathway from lysine and methionine. In contrast to humans, bacteria possess enzymes to catabolize carnitine. Intestinal bacteria degrade most of the orally supplemented carnitine. Carnitine system: A Tool for Understanding Functioning and Dysfunctioning Organs by System Biology 10 genotype/phenotype Mendelian characters Complex biological functions derive from the non-linear interactions of hundreds or thousands of gene products that take place in a specific spatio-temporal order, interactions that are strongly modulated by the environment SYSTEMS BIOLOGY is the new scientific approach to understanding biological complexity and hence to connecting reductionistic analysis with description and prediction of biological functions 4 Modeling DOES NOT WORK! 6 Two approaches for systems biology Global description of cellular function Global functional analysis Bottom-up Identification and wiring of modules Pathways in modules Pathways in networks Modeling of pathways Top-down Analysis of single components INVENTORY OF BIOMOLECULES 7 Definition by medical dictionary CARNITINE Coenzyme of fatty acid oxidation and acetyl transfer; often designated vitamin BT, due to its vitamin role in Tenebrio sp. Present in high concentrations (5% dry weight) in meat extracts (latin carnis =meat). Tenebrio molitor ● 75% provided in diet; 25% synthesized mainly in liver meat, poultry, fish & dairy products 70 – 80% of dietary intake is absorbed O CH3 + CH3 N CH3 CH2 CH OH - CH2 C O L-Carnitine Content of Selected Foods Food Serving L-Carnitine (mg) Beef steak 3 ounces* 81 Ground beef 3 ounces 80 Pork 3 ounces 24 Canadian bacon 3 ounces 20 Milk (whole) 8 fluid ounces (1 cup) 8 Fish (cod) 3 ounces 5 Chicken breast 3 ounces 3 Ice cream 4 ounces (1/2 cup) 3 Avocado 1 medium 2 American cheese 1 ounce 1 Whole-wheat bread 2 slices 0.2 Asparagus 6 spears (1/2 cup) 0.2 An average omnivorous diet provide 2 to 12 μmol of carnitine per kilogram of body weight per day, in contrast to strict vegetarians consuming less than 0.1 μmol of carnitine per kilogram of body weight per day. *A 3-ounce serving of meat is about the size of a deck of cards. L-Carnitine - The daily requirement Supply Biosynthesis: 15 – 20 mg / day (minimum requirement) Nutrition: 2 – 50; ~100 mg / day (meat) Elevated Requirement: Strenuous exercise, diseases, pregnancy, etc. Excretion Prevailing values: 15 – 60 - 120 mg/day Intensive exercise: > 120mg/day Balance Equalized: Not equalized: excretion = supply excretion > supply Consequence: L-Carnitine Deficiency In contrast to microorganisms (i.e., Pseudomonas sp., Acinetobacter sp., Enterobacteriaccae) mammals lack the enzymes which are responsible for the degradation of carnitine Carnitine homeostasis in mammals is maintained by a combination of absorption of carnitine from dietary sources, a modest rate of endogenous synthesis, efficient reabsorption from the glomerular, and mechanisms present in most tissues that establish and maintain substantial concentration gradients between intracellular and extracellular carnitine pools. 18 Carnitine: The Endogenous Synthesis ● 25% synthesized in different organs (The rate of carnitine biosynthesis in humans is estimated to be about 1.2 μmol per kg body weight per day) - liver (major site) - kidneys - brain O CH3 + CH3 N CH3 CH2 CH OH - CH2 C O Some proteins modify lysine to trimethyllysine using Sadenosylmethionine, (SAM) as the methyl donor to transfer methyl groups to the ε-amino of the lysine side chain. Hydrolysis of proteins containing trimethyllysine provides the substrate for the subsequent conversion to carnitine. Scheme of carnitine metabolism on the organism level with the indication of carnitine’ synthesis sites. Nutrigenomics 25 The fate of fatty acyl CoA. Fatty Acid In the fed state, fatty acyl CoA is utilized for triglyceride synthesis, and fatty acid oxidation is dormant. With fasting, fatty acyl CoA is diverted into the oxidative pathway with a fall in conversion to triglycerides. 26 Nutrigenomics Activation of PPAR by fibrates or nonesterified fatty acids released from adipose tissues during fasting causes upregulation of a set of genes involved in fatty acid catabolism including mitochondrial CPT I and CPT II. Upregulation of these genes is mediated by binding of activated PPAR/RXR heterodimers to PPRE present in and around the promoter of those genes. Consequently, in the liver, where PPAR is most abundant, β -oxidation, is dramatically increased as a consequence of the increased expression of PPAR target genes. The resulting increase in β -oxidation of fatty acids enhances the supply of acetyl-CoA used for the generation of ATP via tricarboxylic acid cycle and for the generation of ketone bodies, which are important energetic substrates for the brain during fasting. Since CPT are rate limiting for β-oxidation of fatty acids, the upregulation of CPT might increase the demand of carnitine in cells with high rates of fatty acid oxidation. Thus, the upregulation of enzymes of carnitine biosynthesis and OCTN2 by PPAR during fasting can therefore be regarded as a means to supply tissues with sufficient carnitine required for transport of excessive amounts of fatty acids into the mitochondrion. 28 Carnitine The transporter(s) Phylogenetic tree of the human transporters of the SLC22 family, including two transporters from rodents that have not been detected in human. Distance along the branches is inversely related the degree of sequence identity. For example, sequence identities are 70% between hOCT1 and hOCT2, 32% between hOCT1 and hOCTN1, and 32% between hOCT1 and hOAT1. Polyspecific transporters for organic cations are indicated in red, transporters for organic cations and zwitterions in yellow, and transporters for organic anions in blue OCTN2 Carrier Predicted topology of SLC22 transporters with functional relevant point mutations. Predicted glycosylation sites on the large extracellular loops of hOCTN2 (Ψ) and predicted phosphorylation on the large intracellular loops (blue) are indicated. Amino acids where point mutations resulted in changes of the substrate selectivity are indicated in yellow. Glutamate 452 in hOCTN2 (pink) is thought to be involved in Na+ binding to hOCTN2. Carnitine is also available from the diet after being absorbed in the intestine mainly by active transport. Absorption of carnitine from dietary sources occurs mainly in the small intestine. Initially, uptake of carnitine from the lumen of the intestine across the apical membrane into the enterocyte is mediated in a sodium-dependent manner (Kato et al., 2006). Mrp3 and Abca1 mediate subsequent movement across the basolateral membrane of the enterocyte and into the circulation (Klaassen and Aleksunes, 2010). Mrp3 and Abca1 are ABC family cellular exporters with broad substrate specificities, and here, the apical absorption may be the rate-limiting step. Carnitine uptake across the apical enterocyte membrane is saturable with kinetics akin to OCTN2-overexpressing cells and can be inhibited by Octn2 inhibitors (Kato et al., 2006). By 4 weeks of age, Octn2-null mice demonstrate atrophic intestinal villi, inflammation, ulcer formation, and gut perforation (Shekhawat et al., 2007). Inflammatory bowel disease has been linked to mutations on a locus on chromosome 5 that localizes to the region where the SLC22A4 (OCTN1) and SLC22A5 (OCTN2) genes exist (5q31). Polymorphisms in OCTN transporters are associated not only with the incidence of inflammatory bowel disease, but also with the pharmacological response to therapy. Interestingly, it has been demonstrated that intestinal bacterial components or soluble factors released by gut bacteria are sensed by the colonocytes that increase the OCTN2 expression. Similar to uric acid absorption, renal carnitine handling is a critical step in maintaining systemic carnitine homeostasis. Under normal homeostasis conditions, carnitine is mainly eliminated by excretion in urine. In healthy humans, the daily excretion in urine is 100 to 300 μmol and the tubular reabsorption in the kidney is 90 to 98%. Carnitine handling represents another potential example of remote sensing and signaling in which homeostasis in remote target organs is regulated coordinately. In the kidney, the SLC22A5 gene product is located on the apical membrane of proximal tubule cells. As for other organic cation substrates, SLC22A5 mediates the absorption of carnitine from renal ultrafiltrate into the proximal tubule epithelial cells. Disturbances in carnitine handling at the kidney level might cause other remote organ dysfunctions in heart, muscle, and placenta. 39 The content of carnitine in brain was observed to be lower than in peripheral tissues, with the exception of hypothalamus (Bresolin et al., 1982), which is known to have an easy access to many substances through fenestrations. This observation may point to the existence of some limitations in carnitine accumulation in the brain. A number of uptake (OCTN2) and efflux (Mrp) transporters for carnitine have been localized to the membranes of brain capillary endothelial cells, while the choroid plexus is rich with drug transporters including Oatps but not OCTN2. BRAIN OCTN2 and OCTN3 have been demonstrated in astrocytes and neurons Skeletal muscle and heart contain over 95% of total body carnitine. Since skeletal muscle and heart lack the ability to synthesize carnitine, it is obvious that carnitine transport is fundamental to supply carnitine to these tissues. 44 The preferential distribution of carnitine to heart and skeletal muscle is mediated by carnitine transporters of the SLC22 family, particularly SLC22A5/OCTN2, and probably OCTs (OCT3) as well as Flipt1 and Flipt2 (also known as CT1/SLC22A15 and CT2/SLC22A16, carnitine transporter 2). Because milk is rich in lipids, it is presumed that carnitine is important in preparing the fetus for a postnatal milk diet. Human OCTN2 protein is localized to the apical membrane of syncytiotrophoblasts (Lahjouji et al., 2004; Grube et al., 2005), and is responsible for delivery of carnitine to the fetus during development. During pregnancy, the use of certain anticonvulsants, able to inhibit carnitine uptake, carries a risk of fetal malformations, including congenital heart disease as well as lip and palatal deformity. It is hypothesized that interference of OCTN1 and/or OCTN2-mediated carnitine uptake across placenta by antiseizure drugs increases the risk of fetal developmental defects. It is noteworthy that carnitine is an essential nutrient for newborn infants because the mechanism of its biosynthesis is not fully developed until later in postnatal development. The primary source of carnitine for a newborn is from the mother's milk (Sandor et al., 1982). The neonatal intestine has an avid carnitine absorption system of solute carriers of SLC and ABC families. Likewise, carnitine is handled by a network of transporters in the mammary gland featuring SLC22A5, SLC22A4, and SLC22A3 (Kwok et al., 2006). The carnitine produced in the mammary gland is excreted into the milk and then is absorbed in the baby's intestine. Mother's milk is the only source of carnitine for the newborn infant and is required for the β-oxidation of fatty acids. This process of carnitine transfer from mother to infant represents a coordinated mechanism of solute handling via SLC and ABC transporters between individuals. ~1 in every 12 babies (8.33%) in the United States is born fullterm with low birth weight (LBW) Global incidence of LBW ~17% 49 Maternal Low Protein Diet Full-term Low Birth Weight babies Fetal Programming Altered Physiology Altered Morphology Kidney; Liver; Gastrointestinal tract Altered absorption/production/excretion of carnitine •Hypertension •Hyperlipidemia •Diabetes Mellitus •Obesity Require altered optimal drug dosage for patient Altered Pharmacokinetics 50 Children who develop type 1 diabetes early in life have low levels of carnitine and amino acids at birth: does this finding shed light on the etiopathogenesis of the disease? M. Locatelli, et al. Conclusion: This is the first study demonstrating that children who develop T1D early in life showed reduced circulating carnitine and amino acid levels soon after birth. Their evaluation in the early neonatal period could represent an additional tool in the prediction of T1D and offer new strategies for possibly preventing the disease as early as from birth. 51 Under normal physiological conditions, the primary site of carnitine production is in the liver. During lactation, the production of carnitine in the mammary gland increases, apparently at the expense of production in the mother's liver, which is also accompanied by reduced hepatic enzymatic and transcriptional activity, as well as solute carrier activities associated with carnitine synthesis and handling (Gutgesell et al., 2009). This complex physiological regulation of carnitine synthesis and handling is necessary to provide the infant with carnitine for βoxidation and energy production to use fatty acids. In this instance of carnitine production, transfer from mother to infant, and carnitine utilization in the infant, all via solute carrier networks, mediates the enzymatic activities between and within cells of the mother and the infant Carnitine and its carrier SLC22A5 mediated sensing and signaling among organs and between mother and infant. Liver is the primary site of carnitine biosynthesis. Circulating carnitine is distributed/transported through its carriers, primarily SLC22A5 to the target organs. The synthesis and distribution of carnitine is regulated physiologically. For example, during lactation, carnitine is preferentially distributed to the nursing mother's breast through increased expression of its carriers at the expense of the liver, because mother's milk is the only source of carnitine for the infant and is essential for the survival/growth of the baby. Carnitine The function The two main functions of carnitine are: 1. the transport of long-chain fatty acids into the mitochondrial matrix for β-oxidation to provide cellular energy, and 2. the modulation of the rise in acyl-CoA/CoA ratio 56 Control of fatty acid oxidation is vested in the carnitine palmitoyltransferase system, which consists of three enzymes: carnitine palmitoyltransferase I (CPT I), carnitine palmitoyltransferase II (CPT II), and carnitine:acylcarnitin e translocase (CACT). 57 Carnitine ● Carnitine regulates levels of acyl-CoA inside cells and promotes the clearence of acyl residues - CoA pools are limited and CoA is needed in other processes (GNG, CAC, Urea cycle, b-ox) - Transfer acyl to carnitine to restore CoA pools so the acyl-carnitine serve as a reservoir of activated acyl groups. In addition, acyl-CoA are toxic compounds. -Promote clearance of accumulating acyl residues facilitating their renal or hepatic excretion. O CH3 + CH3 N CH3 CH2 CH OH - CH2 C O Urea Cycle regulated step excreted in urine *emphasis on green text added To CAC carbamoyl phosphate synthetase-I activates acetyl-CoA + glutamate N-acetylglutamate + CoA N-acetylglutamate synthetase ● Without restoration of CoA pools, ● Acetyl-CoA levels drop ● N-acetylglutamate (NAG) will not be made ● CPS I will not be activated and so urea cycle will not proceed ● NH4+ builds up Carnitine and Membrane Integrity Long-Chain Fatty Acids Carnitine CPT1 Protein Acylation Long-Chain AcylCarnitines Acyl-carnitine represents a reservoir of acyl-CoA units at no ATP cost. Membrane Repair Carnitine and plasmamembrane repair • • • • • Oxidation of a polyunsaturated fatty acid esterified in membrane phospholipid (PLP) generates a phospholipid hydroperoxide (PLP-OOH) The deacylating activity of a phospholipase A2 (PLA) hydrolizes the fatty acid hydroperoxide (FA-OOH), that is reduced by a glutathione peroxidase (GSH-Px) to the non-reactive FA alcohol (FA-OH) The lysopholipid acyl-CoA transferase (LAT) regenerates the original PLP avoiding accumulation of the toxic lysophospholipid (LPL). LAT activity requires acyl-CoA synthetized by the ATP-dependent enzyme acyl-CoA synthetase (ACS) Carnitine palmitoyltransferase (CPT), allows the ACS-LAT cycle to keep the acyl-CoA/free CoA ratio constant. Indeed, given the kinetic properties of CPT, any alteration of the acylCoA/free CoA ratio would be promptly buffered by the fully reversible and mass 62 action sensitive CPT reaction. Membrane visco-elastic properties • • • Major determinants of the viscoelastic properties of red blood cell membrane are the cytoskeletal network and membrane phospholipid bilayer. LC may improve the visco-elastic properties of red blood cells by exerting a stabilizing effect on the membrane via interaction with certain cytoskeletal components [spectrin/actin as indicated in the figure by the red circle]. LC would also affect the viscoelastic properties of red blood cell membrane by strengthening the polar head packing of membrane phospholipids 63 Learning carnitine function by carnitine deficiency diseases. 64 Carnitine deficiency can be characterized by low plasma and tissue carnitine concentrations and can be defined as a decrease of intracellular carnitine, leading to an accumulation of acyl-CoA esters and an inhibition of acyl-transport via the mitochondrial inner membrane. 65 Two forms: systemic carnitine deficiency with low carnitine levels in plasma and the affected tissues, and muscle carnitine deficiency, with low carnitine concentration restricted to muscle. Animal model: Juvenile Visceral Steatosis Mouse (severe lipid accumulation in the liver, hyperammonemia, hypoglycemia, cardiac hypertrophy, mitochondrial abnormalities in skeletal muscle and progressive growth retardation). Point mutation from CTG to CGG (leucine to arginine at amino acid position 352) in the mouse homologue of OCTN2 carnitine carrier. 66 A decrease of carnitine levels in plasma or tissues, may be associated with genetically determined metabolic conditions, acquired medical conditions, or iatrogenic states. Disorders of the carnitine cycle or disorders of fatty acid beta-oxidation can cause secondary carnitine deficiency via several mechanisms. Block in fatty acid oxidation contributes to the accumulation of acylCoA intermediates. Transesterification with carnitine leads to the formation of acylcarnitine and the release of free CoA. These acylcarnitines are excreted readily in the urine with increased carnitine losses in the urine and systemic secondary depletion of carnitine. 67 Other genetic conditions that are associated with Fanconi syndrome (eg, Lowe syndrome, cystinosis) may present with secondary carnitine deficiency because of increased renal losses of carnitine. Lysinuric protein intolerance is associated with an increased excretion of lysine in the urine and decreased carnitine synthesis. Cirrhosis or chronic renal failure may impair the biosynthesis of carnitine. Diets with low carnitine content (eg, lacto-ovo–vegetarian diet) or malabsorption syndromes may cause secondary carnitine deficiency Preterm neonates are at risk for developing carnitine deficiency because they have impaired reabsorption of carnitine at the level of the proximal renal tubule and immature carnitine biosynthesis. Iatrogenic causes of secondary carnitine deficiency include several drugs such as valproate. Conditions of increased catabolism present in patients with critical illness. 68