* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download et al. - Deep learning for chemoinformatics
Toxicodynamics wikipedia , lookup
NMDA receptor wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Prescription costs wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Drug interaction wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacokinetics wikipedia , lookup
DNA-encoded chemical library wikipedia , lookup
Orphan drug wikipedia , lookup
Psychopharmacology wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
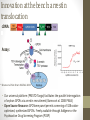
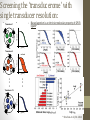
The Thicket of Challenges in GPCR Molecular Pharmacology: Paradigm shifts bring new opportunities and new hurdles Ryan T. Strachan, PhD Goals: • Convey my enthusiasm about the current and future states of GPCR Drug Discovery • Includes the first presentation of a novel screening strategy aimed at studying the unexplored pharmacology of GPCRs (screening the ‘transducerome’) • Initiate a discussion about how to best apply computational methods to facilitate GPCR drug discovery (if at all) • Establish strategic collaborations to facilitate the generation of robust and predictive Deep Learning methods The role of the Molecular Pharmacologist in GPCR Drug Discovery: • Concerned with the study of drug action on a molecular and chemical level • Seek to discover and validate new therapeutic strategies to improve human health • Draw from ideas across multiple fields of study: • • • • Biochemistry Chemistry Physiology Computer Science • • • • Clinical Medicine Mathematics Engineering Statistics GPCRs are key mediators of cell signaling: * Rajagopal et al. Nat Rev. Drug Discov. 2010 • ~800 receptors transduce endogenous and exogenous signals from diverse ligands (photons, odorants, tastants, hormones, neurotransmitters, lipids, etc…) • Large variety of signal transducers (17 different Gα subtypes and 4 arrestins) GPCRs as key drivers of human health and disease: • GPCRs are active in just about every organ system and present a wide range of opportunities as therapeutic targets • Cancer • Obesity • Cardiac dysfunction • Inflammation • Diabetes • Pain • CNS disorders * Courtesy of Tudor Oprea Classic theories give way to new paradigms: • As key drivers of human (patho)physiology GPCRs have been widely studied 1900’s - ‘Chemoreceptors’ and mathematical models of signaling added order to chaos 19701990’s - Advent of functional assays, radioligand binding assays, and the cloning of G proteins and GPCRs paved the way for biophysical and structure-function studies 2000 - Sequencing of the human genome revealed the full complement of GPCRs, including the identification of orphan GPCRs 1990’spresent - Advances in assay technology revealed that GPCR agonists disproportionately activate numerous cellular pathways (ie., biased agonism) via unique receptor conformations 2007present - Advances in GPCR crystallography and computational approaches are ushering in a Golden Age of Molecular Pharmacology, launching the field of structure-based drug discovery Not so fast, we have a long way to go…. Orphan/understudied GPCRs: a treasure trove of drug targets • We possess a very superficial view of how GPCRs function in normal and disease states • ~40% of non-olfactory GPCRs are understudied from chemical and biological perspectives (large green circles) * Roth and Kroeze JBC 2015 Opportunities presented by orphan GPCRs: • Well-characterized GPCRs play key roles in (patho)physiology, therefore orphan/understudied GPCRs have untapped therapeutic potential • Small molecule receptors: • G-21 (5-HT1A serotonin) • RGB-2 (D2 dopamine) • Neuropeptide receptors: • ORL-1 (OrphaninFQ/Nociceptin) • HFGAN72 (Orexin1) • GPR10 (Prolactin-releasing peptide) • APJ (Apelin) • GHSR (Ghrelin) • GPR54 (Kisspeptin/metastin) • GPR73a/b (Prokineticin) • GPR154 (Neuropeptide S) * Civelli et al. Ann. Rev. Pharmacol. Toxicol. 2013 The challenges presented by orphan GPCRs: • Finding tool/endogenous molecules is hard! Interrogating orphan GPCRs en masse in a parallel and simultaneous fashion is currently technologically and economically unfeasible. • Hurdle 1: Uncertainty about which signaling pathway to quantify • Functional assays have typically used readouts that depend on the native or forced coupling of GPCRs with different G proteins, (e.g., Gs, Gi, Gq, G12 or G13) • What about the remaining 12 or so G proteins? • Hard to test all in parallel, run the risk of missing active compounds • Hurdle 2: Chemical diversity-which class of compounds to screen? • Large libraries of diverse chemotypes is preferred Innovation at the bench: arrestin translocation cDNA: Assay: * Kroeze et al. Nat. Struct. Mol Biol. 2015 • Our universal platform (PRESTO-Tango) facilitates the parallel interrogation of orphan GPCRs via arrestin recruitment (Barnea et al. 2008 PNAS) • Open Source Resource: GPCRome panel permits screening of 328 codonoptimized, synthesized GPCRs. Freely available through Addgene or the Psychoactive Drug Screening Program (PDSP) Integrating physical/computational methods to overcome the hurdles of orphan GPCR screening: • Addressed the issue of chemical diversity by integrating physical/computational methods to facilitate tool molecule identification from tens of millions of virtual compounds • Part of a larger discovery effort by the NIH to ‘Illuminate the Druggable Genome (IDG) (https://commonfund.nih.gov/idg/index) • Develop novel, scalable technologies to shed light on the ‘dark matter’ of the human genome in an effort to identify new biology and new therapies • Ion channels • Nuclear receptors • Kinases * Opportunities to mine these datasets via Deep Learning? Integrated workflow: * Using validated screening data to inform the modeling and then cycling between computational prediction and experimental validation has been a key component to success Integrating physical and computational approaches identifies novel chemical matter to reveal new biology: • GPR68 (Huang et al. Nature. 2016) • Proton-sensing GPCR, understudied and lacks tool molecules • Identified a small molecule positive allosteric modulator (PAM), Ogerin • GPR65 (Huang et al. Nature. 2016) • Proton-sensing GPCR with 37% identity to GPR68, understudied • Identified an allosteric agonist and a negative allosteric modulator (NAM) • MRGPRX2 (Lansu et al. Nat. Chem. Biol. in review) • Understudied primate-exclusive GPCR associated with pain and itch • Identified a selective submicromolar agonist tool compound A second major paradigm shift is ‘biased agonism’, which is revolutionizing how we target GPCRs with drugs Biased agonism supplants classic concepts of efficacy: • The “two-state” model postulates an inactive (R) conformation of the receptor in equilibrium with an active (R*) conformation • The “multi-state” models posits that receptors exist in multiple ligand-specific active conformations, each of which possesses varying abilities to activate downstream signaling pathways * Rajagopal et al. Nat Rev. Drug Discov. 2010 Opportunities presented by biased agonism: • Biased agonism can be exploited to target therapeutic pathways and spare those responsible for on target adverse effects - GPCRs μ-opioid receptor Κ-opioid receptor PTH1R GPR109A AT1R β1AR β2AR β2AR D2R Therapeutic Bias - G protein - G protein - Arrestin - G protein - Arrestin - Arrestin - Arrestin - G protein - Arrestin Indication - Pain - Pain - Osteoporosis - Lipid homeostasis - Cardiovascular disease - Cardiovascular disease - Cardiovascular disease - Asthma - Antipsychotic Fulfilling the therapeutic promise of biased agonism: • Limit case: Gi-biased μ-opioid-receptor agonists (PZM21 and TRV130) achieve separation of the analgesic properties of opioids from the arrestinmediated side effects of respiratory depression and addiction. * Manglik et al. Nature. 2016 The challenges presented by biased agonism: • Hurdle: Screening for biased agonists is not straightforward and requires a reference agonist • It is difficult to extracting meaningful information about agonist efficacy from complex cellular assays with varying degrees of signal amplification • Analytical efforts to address this have been hotly debated, yet effective • Transduction coefficients (tau/KA) (Kenakin et al. ACS Chem. Neurosci. 2012) • Emax/EC50 (equiactive comparison) (Figueroa et al. J. Pharmacol. Exp. Ther. 2009) • Tau values (pharmacologic) (Rajagopal et al. Mol. Pharmacol. 2011) Signal amplification changes the location of CRCs: • Scenario where two agonists (e.g., full agonist in red and partial agonist in blue) are tested under varying degrees of signal transduction efficiency (amplification) Hi amplification Low amplification • Potency (EC50) and efficacy (Emax) values change drastically depending on amplification • Very misleading for detecting bias across assays with disparate amplification • Potentially misleading when used in training sets (Rajagopal et al. Mol. Pharmacol. 2011) Amplification turns antagonists into partial agonists: Low amplification Hi amplification Buffer Isoproterenol Procaterol Alprenolol 125 100 cAMP (% ISO Max) cAMP (% ISO Max) 100 Buffer Isoproterenol Procaterol Alprenolol 125 75 50 25 0 75 50 25 0 -14 -12 -10 -8 Log [ligand], M -6 -4 -14 -12 -10 -8 -6 -4 Log [ligand], M • At endogenous β2AR receptor expression levels alprenolol is an antagonist • Overexpressing the β2AR turns alprenolol into a partial agonist Exercise caution when using databases: * Roth and Kroeze JBC 2015 • In silico approaches that take advantage of large databases employing any number of different assays • Despite these issues, we and our collaborators have successfully predicted novel GPCR targets for known drugs and have designed novel drugs targeting GPCRs entirely in silico If cellular assays pose such a problem, then why don’t we bypass them? Bypassing the need for cells: quantifying signaling in vitro *Accomplished by viewing GPCRs as allosteric machines • Intrinsic efficacy (ε) of classic theory is equal to the energetic effect that drives formation of an active ternary complex (α) * Onaran et al. Trends Pharmacol. Sci. 2014 * Onaran and Costa Nat. Chem. Biol. 2012 *Suggests that we can quantify signaling through different transducers in vitro by measuring cooperativity between the ligand and transducer (i.e., by shifts in agonist affinity) Quantifying signaling in vitro is nothing new: • Coincident with development of the Ternary Complex Model (TCM) it was shown that shifts in agonist affinity (molecular efficacy) correlate intrinsic efficacy in cells *De Lean et al. JBC. 1980 *Kent et al. Mol. Pharmacol. 1980 Screening the ‘transducerome’ with single transducer resolution: [ligand] Transducer 2 T T * 100 50 0 no T %Bound unfused Biased agonism is an intrinsic molecular property of GPCR 150 ligands fus ion 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 T C curve shift integral T T B %Bound Transducer 1 transducer fused ... [ligand] ... Transducer 19 T T T %Bound A [ligand] * Strachan et al. JBC. 2014 Goal: Mine the unexplored pharmacology of GPCRs for new modes of signaling bias: * T T fused ... ... %Bound Transducer 19 0 7 8 9 10 11 12 13 14 15 16 17 18 19 Transducer transducer • This would require patterns to be extracted from complex data sets • e.g., transducerome shifts, clinical endpoints, gene expression, and behavioral data Transducer 19 T T 0 transducer [ligand] T 50 50 Unfused T 100 no %Bound unfused * 100 no [ligand] Transducer 2 Transducer 2 150 curves between Areacurve shift integral C 150 fus ion 1 2 3 4 5 6 7 8 fus 190 i1o1n 12 131 142 153 164 17 185 196 T C ... T T B %Bound Transducer 1 ... A B Transducer 1 curve shift integral A [ligand] • To our knowledge no one is thinking on this scale Summary: • The field of GPCR Molecular Pharmacology is rapidly changing, reinvigorated by paradigm shifts related to the notions that: • A large fraction of receptors are understudied or ‘orphaned’ • Biased agonism is a property of GPCR ligands • Paradigm shifts afford both numerous opportunities AND challenges • We have a long way to go in order to fully exploit this current Golden Age of Molecular Pharmacology • I am confident that advances in crystallography and computational medicinal chemistry will help to accelerate discoveries Opportunities for Deep Learning to facilitate GPCR drug discovery: • Identification of novel chemical matter (empirical approaches are too slow) from virtual screening campaigns • Tool molecules for illuminating understudied/orphan GPCRs • Biased agonists (facilitated by biased GPCR structures, e.g., bound by different agonists, different ternary complexes, Nbs, etc…) • Mine complex clinical, transcriptomic, proteomic, and ‘transducerome’ datasets at high levels of abstraction to uncover novel modes of therapeutic bias • Step closer to the NIH notion of ‘Experimental Medicine’ as it relates to fully characterizing drug actions before they advance to large clinical trials The call to collaborate: successful integration of wet bench pharmacology and computation Empirical screens *Collaboration has been essential Computation/ prediction Goal: Establish a project devoted to generating the optimal AI training set for GPCR ligand discovery Target: Well-characterized GPCR family with multiple crystal structures (e.g., opiate receptors) Ligands: Large library containing multiple chemotypes, with substantial SAR within each chemotype Data (raw and corrected): - Binding affinities (Ki’s through the Psychoactive Drug Screening Program) - Efficacy values (tau for standard assays such as Ca2+ release, cAMP, arrestin recruitment; use the Psychoactive Drug Screening Program ) - Molecular efficacy values from ‘transducerome screening’ Thank you! Questions?