* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
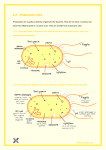
Optimization of E. coli culture conditions for efficient DNA uptake by electroporation 1 Introduction Bacterial transformation is a good breakthrough in the field of molecular biology for cloning purposes For carrying out transformation the basic requirements are Foreign DNA Vector Cells having capability to take up exogenous DNA Transformation technique • Further transformation results could be checked out initially by Antibiotic selection Blue/white screening 2 Blue/white screening(Bacterial colonies transformed with pUC18) White colonies (contain recombinant DNA molecules) blue colonies (contain non-recombinant DNA molecules) 3 How it will be confirmed that either your transformation experiment is successful or not Restriction digestion PCR amplifications Sequencing 4 Gene transfer results can be stable or transient Transient : DNA is not integrated into host chromosome. DNA is transferred into recipient cells in order to obtain a temporary but high level of expression of target gene Stable: The transferred DNA is integrated into chromosomal DNA and the genetics of cell is permanently changed 5 Thus for a bacterial cell to take up DNA from its surroundings, it must be in a special physiological state called competence Competency is the ability of bacterial cells to take up exogenous DNA from close vicinity OR Competent cells are those that possess more easily altered cell walls that DNA can be passed through easily Competence is distinguished into Natural competence Artifical Competence 6 Natural competence Bacteria are able to take up DNA from their environment (exogenous DNA) in three ways; Conjugation Transformation Transduction 7 Natural competence is highly regulated in bacteria, and the factors leading to competence vary among genera For some genera, only a portion of the population is competent at any time; for others, the entire population gains competence A series of competence proteins is produced, which have some homology but differ in the Gram negative and the Gram positive bacteria. Once the DNA has been brought into the cell's cytoplasm It may be degraded by cellular nucleases If it is very similar to the cells own DNA, enzymes that normally repair DNA may recombine it with the chromosome. Transformation can occur naturally in Streptococcus, Bacillus Acinetobacter, Neisseria, and Pseudomonas 8 Artifical competence There are two methods to induce artificial competence Chemical Physical Chemically induced competence followed by transformation is a commonly used technique to introduce plasmids or other DNA fragments into Escherichia coli. In chemical method bivalent cations (Ca+2, Mg+2, Ba+2, Rb+ and Sr+2) are chiefly used to induce competence in cells (Huang and Reusch, 1995) 9 Contd… Beside these many other chemicals e.g. EDTA (Williams et al., 1996), Polyethylene glycol (Himeno et al., 1984) Osmotic agents including Sucrose (Antonov et al., 1993) Glycine (Holo and Nes, 1989) Sorbitol and manitol (Xue et al., 1999) These chemicals not only increase the chances of transformation but it also improves the chances of cell survival when present in high concentrations in the electroporation medium 10 Alteration in the permeability of the membranes allows DNA to cross the cell envelope of E. coli which is composed of an outer membrane, an inner membrane, and a cell wall. The outer membrane of E. coli can be understood by application of the fluid mosaic model for membranes and is composed of phospholipids, proteins, and lipopolysaccharides Many channels or zones of adhesions are formed by the fusion of the outer membrane and the inner membrane through the cell wall layer 11 Although the transformation mechanism is not known, previous studies indicate that The channels between outer and inner membrane allow for the transport of DNA molecules across the cell membrane. The negative charges of the incoming DNA, however are repelled by the negatively charged portions of the macromolecules on the bacterium’s outer surface. The addition of CaCl2 serves to neutralize the unfavorable interactions between the DNA and the polyanions of the outer layer 12 The DNA and competent cells are further incubated on ice for thirty minutes to stabilize the lipid membrane and allow for increased interactions between calcium ions and the negative components of the cell The reaction mixture is then exposed to a brief period of heat-shock at 42oC Alters fluidity of membranes 13 Methods to induce artifical competence There are two methods to induce artificial competence Chemical Physical Electroporation is a most popular physical process of producing transient pores in outer membrane of living cells by subjecting them to a rapidly changing high strength electric field. 14 Contd.. Electroporation is enhanced by several factors, Wu et al. (2010) reported that careful control of (i) Cell growth phase (ii) Electroporation cell number (iii) Cell desalination with glycerol (iv) Amount of transforming DNA may enhance transformation efficiency of E. coli up to 1010 transformants/µg DNA (10-40pg) 15 Reasons for preference of physical method over chemical method of competent cell preparation. Method of preparing electrocompetent cells is preferred over the chemical method for transferring exogenous DNA into bacteria, because of its High transformation efficiency that is suitable for regular plasmid, ligated cDNA, and genomic DNA transformation Suitability of using less transforming DNA amount for high transformation efficiency, which is beneficial for rare and/or hard-toobtain gene DNA Transformation irreversibility compared to chemical transformation method (Kurien and Scofield, 1995). 16 Limitations During conventional electrocompetent cell preparation, extra-sodium chloride is normally used as a major ion source in the medium (about 1% in LB medium). The combination of chemical-chemical, chemical-physical and physical- physical methods improves the transformation manifolds and is used in laboratories worldwide. Bukau et al. (1985) suggested that the growth medium supplemented with sucrose facilitates DNA uptake Transformation efficiency of E. coli may also be enhanced by the use of simplest amino acid, Glycine (1% w/v) in growth medium reported by Akhtar et al. (1999). 17 Ahmed et al. 2014 utilize LB and SOC media and their supplemented combinations (Sucorse and Glycine) to prepare electro-competent cells of E. coli DH5α at temperature 25 and 37 °C which were then subjected to electroporation. For carrying out their study they utilize DH5 α strain of E. coli The plasmid pENTRTM/D-TOPO® gateway entry vector of size 2580 bp was used to produce recombinant vector pENTR/D-TOPO-RGLP1 of 3255 bp by ligation of 675 bp long fragment. The vector harbors kanamycin resistance gene as a selection marker. Media combinations (LB, LB-S, LB-SG, LB-G) (SOC, SOC-S, SOCSG, SOC-G) 18 What methodology they adopted Growth kinetics of E. coli DH5α was studied in all the combinations of media at temperatures 37 ºC and 25 ºC Electrocompetent cells of E. coli DH5α was prepared by following protocol of Sambrook and Russel (2001) with certain modifications, In this study competent cells were prepared in media combinations of LB, LB-S, LB-SG, LB-G, SOC, SOC-S, SOC-SG, SOC-G at two different temperatures 25 ºC and 37 ºC Electroporation of competent cells PCR confirmation of plasmid 19 The results showed that cells grown in SOC media showed high growth rates and increased transformation efficiencies as compared to LB. Results of growth kinetics show that SOC-S and SOC-SG are better media combinations for achieving required density of 0.3- 0.45 for preparing competent cells at both temperatures in less time as evident from the comparison of results depicted in Figures 1, 2, 3 and 4. 20 21 However efficient growth kinetics of E. coli is not an indication that it will transform efficiently as it has been reported by Wu et al. (2010) that transformation efficiency is inversely proportional to cell population size The increased growth rate in presence of SOC media might be due to the fact that SOC is a glucose containing nutrient rich media, and glucose is a readily metabolizable sugar (Tu et al., 2005) 22 In contrast, glycine when added to growth media may interfere with the biosynthesis of membranes by replacing L- and Dalanine from peptidoglycan with glycine and thus reducing cell growth (Kaderbhai et al., 1997) As glycine is metabolized into acetate while acetate is an inhibitor of bacterial growth in this way it reduces growth rate of E. coli (Luli and Strohl, 1990). 23 24 Further increased transformation efficiencies were observed by Ahmed et al. 2014 in SOC media as compared to LB media which has been speculated to be due to Presence of bivalent cation (MgCl2) in SOC media which helps to facilitate DNA uptake In all combinations tested highest transformation efficiencies were observed for the cells grown in SOC-SG at 25 ºC i.e. 3.56 × 109 cfu/µg of DNA might be due to Mass flow of sucrose along with transformation mixture Structural impariments 25 In addition to media combinations of osmotic agents temperature, was also tested as parameter for enhancing electroporation efficiency An ehanced electroporation rate at lower temperature (25 ºC) as compared to optimum temperature for DH5α growth as demonstrated in Table 2 When cells are grown in temperature lower then optimum temperature (37 ºC), proportion of unsaturated fatty acids in their membranes increases (Gill and Suisted, 1978) which increase fluidity and can interfere membrane functions (Kuo et al., 1990) thus making membrane more permeable to DNA. 26 27 Conclusions This study supports previous findings that osmotic agents and lower temperature are imperative factors in enhancing DNA uptake efficiency Ahmed et al. 2014 concluded that efficient culture media for increasing number of transformants per µg DNA is SOC media, supplemented with Sucrose and glycine as compared to LB. The investigation reinforces the notion that even limited size/ low budget molecular biology laboratories could efficiently prepare highly efficient electrocompetent cells with transformation efficiencies of 3.56 x 109cfu/µgDNA. 28 Reference Irfan Ahmed, Tehseen Rubbab*, Farah Deeba, Syed Muhammad Saqlan Naqvi. Optimization of E. coli culture conditions for efficient DNA uptake by electroporation. Turk J Biol. (2014), 38:568-573. 29 Thanks 30