* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Induced pluripotent stem cells - The Stem Cell Training Course
Cytokinesis wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cell encapsulation wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
Somatic cell nuclear transfer wikipedia , lookup
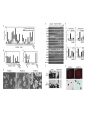
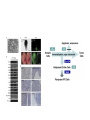
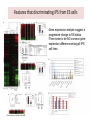
Induced pluripotent stem cells – the science and technology Albert Lasker Basic Medical Research Award, 2009 Chi-Wei Lu, Ph.D. Assistant Professor, RWJMS/UMDNJ Director, Human Embryonic Stem Cell Facility Pluripotent stem cells and tissue replacement therapy • • • • • Stemcells.nih.gov Cell replacement therapy is to restore the functional lost of a defined cell type: including diabetes (lost of b-islet cells), immunodeficiency (loss of cell mediated immune response), Parkinson’s disease (lost of dopaminergic neuron). The source of stem cells are limited to donor tissue with tissue type matched with the patients. Most of the tissue stem cells have minimal proliferation in vitro, limited for being a sustainable source as donor tissue. Pluripotent stem cells can self-renew and differentiate into various types of mature tissue in vitro, is considered an ideal source for donor tissues. Blastocyst derived human embryonic stem cells requires banking to a large number in order to match the need of the majority of people. Embryonic stem cell, SCNT-embryonic stem cells and other reprogramming A patient fibroblast reprogrammed into the pluripotent state would provide tissues that match all HLA antigen to the patient. • Much hope has been imposed on the development of somatic cell nuclear transfer (SCNT) technology, which allows to re-program patient fibroblasts into pluripotent stem cells. • SCNT involves highly specialized technique and is limited to the availability of oocytes as the reprogramming vehicle. The successful derivation of SCNT-ES cells have been reported in primate (Bryne and Mitalipov, Nature 2007). Cellular Reprogramming • Cell-cell fusion experiments suggest that the ES cell dominate the hybrid cell phenotype. (Clarke and Frisen, Science 2000; Terada and Scott Nature 2002; Ying and Smith Nature 2002; Cowan and Eggan Science 2005). • The hybrid ES cells possess all properties of pluripotent stem cells – including teratoma formation, selfrenewal, yet contains a cell nucleus from an exogenous cells. • Partial reprogramming have been observed in ES cytoplast and ooplasm fusion. (Strelchenko and Verlinsky, Reprod Biomed Online 2006, Bru, Wilmut and Blow, Exp Cell Res 2008, Wang and Cibelli, 2009). Cowan and Eggan, Science, 2005 Pluripotency induction: cell fate change by expression of transcription factors • Ectopic expression of transcription factors can reprogram cells into another lineage: Gata1 convert lymphoid/myeloid cells into Mega/Erythroid cells (Iwasaki and Akashi, Immunity 2003) • Takahashi and Yamanaka reported that pluripotent stem cells can be established by 4 transcription factors selected from a list of 24 mouse ES enriched genes: Oct4, Sox2, Klf4 and Myc. (Takahashi and Yamanaka, cell, 2006) • Reports from the laboratories of Thomson (Yu and Thomson, Science 2007), Yamanaka ( Takahashi and Yamanaka, Cell 2007), Daley (Park, Nature 2008) and Plath (Lawry and Plath, 2008) reproduced the iPS phenomenon in human dermal fibroblasts by ectopic expression of four embryonic stem cell specific transcription factors. Zaehres and Scholar, Cell 2007 The generation of induced pluripotent stem cells – the Takahashi and Yamanaka paper, Cell, 2006 24 candidate factors: Ecat1, Dpp5(Esg1), Fbx015, Nanog, ERas, Dnmt3l, Ecat8, Gdf3, Sox15, Dppa4, Dppa2, Fthl17, Sall4, Oct4, Sox2, Rex1, Utf1, Tcl1, Dppa3, Klf4, b-cat, cMyc, Stat3, Grb2 Transcription factors are delivered by retroviral vectors and the colonies became visible by day 16 The induced pluripotency phenomena in human cells -Takahashi and Yamanaka (Cell, 2007) : retroviral vector (pLAT-A) with Oct4, Sox2, Klf4 and c-Myc -Yu and Thomson (Science, 2007): lentiviral vector (pSIN) with Oct4, Nanog, Sox2 and Lin28 -Park and Daley (Nature 2008): retroviral vectors (pMIG): Yamanaka factors with SV40T and hTert -Lowry and Plath (PNAS 2008) : retroviral vectors (pMX): Yamanaka factors Colonies can be identified after 14 days, select by colony morphology. Validating human iPS colonies during production - While ES-like colonies can be observed, most of colonies are resulted from incomplete reprogramming. -“early” colonies may only express a part of the reprogramming factors without turning on endogenous pluripotent genes. -”later” colonies, emerge about 3 weeks after transduction, are phenotypically identical to ES cells. The criteria to define a bona fide iPS cell • Non viable staining : Positive for alkaline phosphatase staining and pluripotency factors including NANOG. • Viable staining for cell surface antigen expression: Tra-1-81(+), Tra-1-60(+), SSEA3(+) SSEA4(+)(Lowry and Plath,PNAS 2008, Chan and Daley, Nat Biotech 2009) • Expressing endogenous pluripotency associated genes, including OCT4, SOX2, NANOG, REX1, FGF4, ESG1, DPPA2/4, hTERT, DNMT3B, etc. (Takahashi and Yamanaka, 2007) Exogenous: cDNA Endogenous: RNA with intron • The ability to differentiate into all three germ lineages in embryoid bodies and form teratoma in immunodeficient animals. • The erasure of imprinting (DNA methylation) at pluripotency gene promoters, including Oct4, Sox2, Nanog etc. Features that discriminating iPS from ES cells -Gene expression analysis suggest a progressive change to ES status. -There seem to be NO common gene expression difference among all iPS cell lines. Chin and Lowry, Cell Stem Cell 2009 Features that discriminating iPS from ES cells • mES cells can chimerize embryos with higher efficiency while the chimerism of iPS is excluded from germ-line. • Microsatellite DNA are polymorphic loci present in genomic DNA uniquely identifies unrelated individuals and cell lines. The specific lengths of microsatellite mark a cell’s origin so an iPS clone can be traced to its fibroblast origin and determine if the contamination from established ES cell lines is present. (Takahashi and Yamanaka, Cell 2007) iPS cells generation in patient fibroblasts • • • • Parkinson’s disease (Wernig and Jaenisch, 2008, Maehr and Melton PNAS 2009). Amyopathic Lateral Sclerosis, (Dimos and Eggan Science 2008) Type I diabetes (Maehr and Melton PNAS 2009) ADA-SCID, SBDS, Gaucher disease, Duchenne and Becker Muscular dystrophin, Parkinson’s disease, Huntington disease, JDM, Down syndrome, Lesch-Nyhan syndrome. (Park and Daley Cell 2008). iPS cells generation from other cell types • • • • • Blood cells (Loh and Daley 2009). B-cells (Hanna and Jaenisch Cell 2008) Blood stem cells (Emiinli and Hochedlinger Nat Genet 2009) Pancreatic b-cells (Stadtfeld and Hochedlinger Cell Stem Cell2008) Hepatic and gastric endoderm (Aoi and Yamanaka Science 2008) Neural stem cells (Kim and Scholar, Nature 2008) Developments toward the “safer” iPS generation Reduced number of transcription factor use: • No myc: Nakagawa and Yamanaka, Nat Biotechnol 2008, Wernig and Jaenisch, Cell Stem Cell 2009 • No Sox2: by adding GSK-3 inhibitor, Zhou and Ding, Stem cell 2009, in neural stem cell, Kim and Scholer Nature 2008 • No Klf4/myc, by addition of Valproic acid : Huangfu and Melton, Nat Biotech 2008 • No Myc and Sox2, by addition of BIX01294 and PD0325901 (Zhou and Ding, Cell Stem Cell 2008). • Klf4 only by adding Kenpaullone (Lyssiotis and Jaenisch, PNAS 2009) Specific pathways: • TGFb inhibitor replace Sox2 and cMyc and induce Nanog (Maherali and Hochedlinger, Curr Biol 2009, Ichida and Eggan 2009 ) • p53 inhibition augments iPS efficiency (Hong and Yamanaka, Nature 2009,Utikal and Hochedlinger Nature 2009, Marion and Blastco Nature 2009, Li and Serrano Nature 2009, Kawamura and Belmonte 2009) • Hypoxia – Yoshida and Yamanaka Cell Stem Cell 2009 • WNT signaling stimulates reprogramming efficiency (Marsonm, Jaenisch Cell Stem Cell 2008) Better vectors: • Drug Inducible vectors: Wernig and Jaenisch, Nat Biotechnol 2008, Hockemeyer and Jaenisch, Cell Stem Cell 2008 • Non-integrating vectors : adenovirus in hepatocyte (Stadtfeld and Hochedlinger Science 2008) • Self-inactivating vectors: Piggy Bac (Yusa and Bradley, Nat Methods 2009) • multi-cistronic vectors: single lentiviral cassette ( Carey and Jaenisch, PNAS 2009, Sommer and Mostoslavsky, Stem Cell 2009) • Vector free (episome Yu and Thomson, Science 2009; direct transfection Okita and Yamanaka Science 2008) • Direct protein induction: poly arginine modification of recombinant protein (Zhou and Ding, Cell Stem Cell 2009), Summary • Reprogramming patient cells into a pluripotent state provides the best matching and the most abundant source for tissue regeneration. • Among all different methods, the most achievable is direct reprogramming, by introducing pluripotency associated transcription factors into primary tissue culture. • Direct reprogramming generates induced pluripotent stem cells that are functionally and phenotypically identical to embryonic stem cells. • “Safer” protocol in generating less tumorigenic induced pluripotent stem cells are rapidly evolving. • More works are needed in order to employ iPS cells for tissue replacement therapeutics.