* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2.3 Carbon Compounds
Carbon sink wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Isotopic labeling wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosequestration wikipedia , lookup
Genetic code wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Proteolysis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
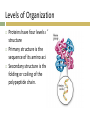
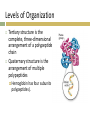
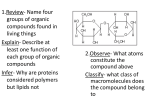
1.Review- Name four groups of organic compounds found in living things Explain- Describe at least one function of each group of organic compounds Infer- Why are proteins considered polymers but lipids not 2.Observe- What atoms constitute the compound above Classify- what class of macromolecules does the compound belong to CH 2 CHEMISTRY OF LIFE 2.3 Carbon Compounds Organic chemistry means the study of compounds that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds In the early 1800s, many chemists called the compounds created by organisms “organic,” believing they were fundamentally different from compounds in nonliving things. The Chemistry of Carbon Have four valence electrons Allows them to form 4 strong covalent bonds with many other elements Living organisms are made up of molecules that consist of carbon and other elements. The Chemistry of Carbon Carbon atoms can also bond to carbon atoms Carbon-carbon bonds can be single, double, or triple covalent bonds Chains of carbon atoms can also form rings. Macromolecules Made from 1000’s or even 100,000’s of smaller molecules Formed by a process known as polymerization Large compounds are built by joining smaller ones together. Macromolecules Monomers The smaller units Polymers When the monomers join together. Macromolecules Sorted into groups based on their chemical composition Four major groups are Carbohydrates, Lipids, Nucleic acids, and Proteins. Carbohydrates Only contain Carbon, Hydrogen, and Oxygen Always the same ration CH2O Sugars are the subunits and may vary in number of carbons it contains To make a carb., hook many sugars together. Simple Sugars Monosaccharides Single sugar molecules Glucose Galactose Fructose Disaccharide Compound made of two single sugars Sucrose Not all sugars are sweet. Uses of Carbohydrates Energy Storage Building. Carbohydrates Store extra sugar as complex carbohydrates. The monomers in starch polymers are sugar molecules, such as glucose. Complex Carbohydrates Large macromolecules formed from monosaccharides are known as polysaccharides. Complex Carbohydrates Starch Energy storage in plants, human food source Cellulose Structure in plants, humans cannot digest Glycogen Energy storage in animals. Lipids Only contain Carbon, Hydrogen, and Oxygen Consists of long Carbon chains with Hydrogens attached Very efficient at storing energy- 2X per mass as carbs. Other Uses of Lipids Cholesterol Hormones Steroids Phospholipids. Lipids Formed when a glycerol molecule combines with compounds called fatty acids. Lipids Saturated Each carbon atom in a lipid’s fatty acid chains is joined to another carbon atom by a single bond Unsaturated At least one carbon-carbon double bond in a fatty acid Polyunsaturated Contain more than one double bond. Lipids Unsaturated fatty acids tend to be liquid at room temperature Saturated fatty acids tend to be solid at room temperature. Nucleic Acids Used for hereditary information Made of Nucleotides Contain a phosphate, sugar, and nitrogen base Nucleotides join together to form Nucleic acids. Nucleic Acids Nucleotides can be joined by covalent bonds to form a polynucleotide, or nucleic acid Two kinds of nucleic acids: Ribonucleic acid (RNA) Deoxyribonucleic acid (DNA). Protein Used for muscle, cell structure, and enzymes Formed using the subunit Amino Acids Always have the pattern N-C-C. Amino Acids 20 different amino acids Plants can make all 20, we can’t Amino group (–NH2) on one end and a carboxyl group (–COOH) on the other. Making a Protein Peptide Bonds Covalent bonds link amino acids together to form a polypeptide. Levels of Organization Proteins have four levels of structure Primary structure is the sequence of its amino acids Secondary structure is the folding or coiling of the polypeptide chain. Levels of Organization Tertiary structure is the complete, three-dimensional arrangement of a polypeptide chain Quaternary structure is the arrangement of multiple polypeptides Hemoglobin has four subunits (4 polypeptides).