* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Claudins, dietary milk proteins, and intestinal barrier regulation
Survey
Document related concepts
Circular dichroism wikipedia , lookup
Protein structure prediction wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein purification wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Transcript
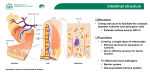
bs_bs_banner Emerging Science Claudins, dietary milk proteins, and intestinal barrier regulation Belinda M Kotler, Jane E Kerstetter, and Karl L Insogna The family of claudin proteins plays an important role in regulating the intestinal barrier by modulating the permeability of tight junctions. The impact of dietary protein on claudin biology has not been studied extensively. Whey proteins have been reported to improve intestinal barrier function, but their mechanism of action is not clear. Recent studies, however, have demonstrated increased intestinal claudin expression in response to milk protein components. Reviewed here are new findings suggesting that whey-protein-derived transforming growth factor b transcriptionally upregulates claudin-4 expression via a Smad-4-dependent pathway. These and other data, including limited clinical studies, are summarized below and, in the aggregate, suggest a therapeutic role for whey protein in diseases of intestinal barrier dysfunction, perhaps, in part, by regulating claudin expression. © 2013 International Life Sciences Institute INTRODUCTION Epithelial monolayers form selective barriers to protect organ systems from the external environment. A critical example is the gut epithelium, which has evolved complex mechanisms not only to allow nutrient absorption but also to defend against entry of infectious agents and foreign antigens into the body. The impact of nutrients on gut epithelial function and, in particular, the gut epithelial barrier, is poorly understood. Molecular mechanisms of nutrient actions in this tissue are also unclear, but recent work has provided new insights into this area. Absorption of nutrients, including minerals, can occur through both transcellular and paracellular mechanisms. Transcellular mechanisms usually involve specific cell-surface transporters or acceptor molecules that allow for selective uptake of molecules that are transported across the cell; this transport is often achieved with the assistance of carrier proteins that are either utilized by the intestinal epithelia or are exported through the basolateral membrane to the systemic circulation. In contrast, paracellular transport involves the movement of molecules, usually across a concentration gradient, between cells. The selectivity of paracellular transport relies on the specific composition of the proteins that form intercellular junctions. This review focuses on paracellular transport and the role of tight junctions in that process.Within an epithelial monolayer, cell-to-cell contacts, known as tight junctions, regulate the flux of water, ions, and proteins across tissue barriers by allowing for the movement of molecules between cells.1 Tight junctions are composed of membrane-spanning proteins, whose components traverse the paracellular space, and scaffolding proteins, which link membrane proteins directly to the actin cytoskeletal network. As shown in Figure 1,2 membrane-spanning proteins, including claudin, occludin, junction adhesion molecule, and scaffolding proteins, including the zona occludens, collectively regulate the permeability of tight junctions.3 Claudins are essential for barrier function by virtue of their critical role in regulating selectively permeable ion channels in tight junctions.4 Recently, the influence of nutrition on the biology of tight junctions and, specifically, on the regulation of activity and level of expression has become an area of investigative interest. Although the effects of nutrients Affiliations: BM Kotler and JE Kerstetter are with the Department of Allied Health Sciences, University of Connecticut, Storrs, Connecticut, USA. KL Insogna is with the Department of Internal Medicine, Section of Endocrinology, Yale University, New Haven, Connecticut, USA. Correspondence: BM Kotler, Department of Allied Health Sciences, University of Connecticut, 358 Mansfield Rd, Unit 2101, Storrs, CT 06269-2101, USA. E-mail: [email protected], Phone: +1-860-486-1996. Key words: claudin, intestinal barrier function, TGFb, tight junction, whey protein 60 doi:10.1111/j.1753-4887.2012.00549.x Nutrition Reviews® Vol. 71(1):60–65 Figure 2 Claudin structure. A. Key features of a typical claudin in the plane of the membrane. Shown are two extracellular loop domains, where extracellular loop 1 (EL1) contains a putative disulfide bond (S-S). Cylinders represent predicted transmembrane alpha-helical domains. Also illustrated are two palmitoylation motifs (P) and the PDZ-binding motif at the extreme C-terminus. B. The four transmembrane alpha-helical domains are depicted as a tightly packed complex. Adapted from Overgaard et al.4 Abbreviation: COOH, C terminus. Figure 1 Structure of tight junctions. Membranespanning proteins such as claudin, occludin, and junction adhesion molecule (JAM) seal the paracellular space between adjacent epithelial cells. Scaffolding proteins, such as the zona occludens, act as adaptors that connect membrane-spanning proteins to the actin cytoskeletal network. Adapted from Ulluwishewa et al. 2 Abbreviations: ZO1/2, zona occludens 1 and 2; ZO1/2/3, zona occludens 1, 2, and 3. and of dietary protein in particular on claudin expression and intestinal barrier regulation have not been studied extensively, they are likely to be important, given the critical role of claudins in regulating the gut epithelial barrier. This review focuses on recent studies that report an effect of whey protein on claudin-4 expression and the impact this has on intestinal barrier function.5 DESCRIPTION AND STRUCTURE OF THE CLAUDIN FAMILY The family of claudin proteins currently consists of 24 members. Claudins were first identified by Furuse et al.6 and derive their name from the Latin word “claudere,” which means “to close.”3 Claudins have a molecular mass ranging from 20 kDa to 27 kDa and are composed of four transmembrane domains, two extracellular loops, a short cytosolic N-terminus, and a longer cytosolic C-terminus that includes a PDZ-binding domain, which is present in nearly all isoforms (Figure 2).4 Extracellular loop 1 (EL1) consists of approximately 52 amino acids, including a conserved cysteine-containing motif,4,7 and is predominantly responsible for the charge-selective permeability of paracellular transport. Extracellular loop 2 (EL2), approximately 16 to 33 amino acids long, is presumed to Nutrition Reviews® Vol. 71(1):60–65 fold into a helix motif and, along with EL1, is critical for claudin-claudin interaction.4 The length of the C-terminus varies between claudin isoforms, from 21 to 63 amino acids, and contributes to isoform-dependent paracellular selectivity.3 The C-terminus PDZ-binding motif facilitates direct interaction between claudins and scaffolding proteins. Not all claudins contain a PDZbinding domain, claudin-12 being an example.8 The N-terminus consists of seven amino acids and has no known function.1 CLAUDIN FUNCTION As noted above, intestinal absorption of nutrients can occur via transcellular or paracellular transport. In contrast to transcellular transport, which usually requires energy expenditure, paracellular transport is passive and depends on an electrochemical gradient. The tight junctions, including the claudin proteins, facilitate paracellular transport by forming ion-selective pores.9 Claudins can be classified as either pore forming or pore sealing. Pore-forming claudins increase paracellular permeability through the formation of channels, whereas pore-sealing claudins reduce paracellular permeability. A wide variety of incompletely understood factors determine whether claudins enhance or reduce paracellular permeability. These include claudin-claudin interactions, which can occur head-to-head between the same claudins on adjacent cells (homotypic interactions) or, much less frequently, between different claudins (heterotypic interactions). Claudins can also interact side-to-side in homomeric or heteromeric interactions4 (see also the citations in Overgaard et al.4). Additionally, claudins may interact with other components of the tight junction, including 61 occludins and zona occludens 1, and can undergo posttranslational modification such as palmitoylation and serine-threonine phosphorylation.4 These complex interactions in the microenvironment of the tight junction, as well as the tissue-specific expression of certain claudins, underlie their varied effects on permeability, which are for the most part poorly understood. Based in part on their molecular size, solutes are thought to cross tight junctions. Solutes less than 4 angstroms (e.g., sodium, magnesium) follow a pathway that carries electrical charge during transport. Increased claudin-2 expression in cultured epithelia increases permeability for solutes less than 4 angstroms. The pathway for solutes greater than 4 angstroms (e.g., mannitol, inulin) carries no electrical charge and is speculated to represent temporary breaks in otherwise continuous tight junctions.10 Charge-selective regulation of claudin proteins is determined by the structural elements in EL1. The negatively charged residues in EL1 strongly influence cation pore formation. For example, the replacement of basic residues with acidic residues in the EL1 domain of claudin-4 increased cation permeability. EL2 collaboration with EL1 seems to be required for proper pore formation.11 CLAUDINS AND INTESTINAL BARRIER REGULATION Several claudins have been reported to have functional roles in the intestine. Claudin-1, -3, -4, -5, and -8 function as pore-sealing proteins, whereas claudin-2 forms chargeselective pores, for example, facilitating the paracellular transport of calcium. Claudin-12 has also been reported to increase paracellular calcium transport in enterocytes.12 Claudin-7 appears to reduce tight-junction permeability, as indicated by studies in knockout mice.13 The role of claudin-15 in regulating intestinal barrier function is unclear, but this claudin seems to have distinct biological actions in enterocytes, where it increases cell proliferation.14 Paracellular permeability can inappropriately increase with disease and age, as well as during periods of stress. Understanding the mechanisms by which claudin proteins regulate the intestinal barrier will provide insight into the pathophysiology of intestinal barrier malfunction as well as identify targets for therapeutic intervention.2 Consistent with this idea, recent studies indicate that manipulation of claudin function in experimental models affects the intestinal barrier. Ewaschuk et al.15 determined that claudin-2 expression is suppressed in T84 cell monolayers (an enterocyte cell model) following 4 hours of treatment with metabolites secreted by the commensal organism Bifidobacterium infantis. Thus, one mechanism by which microorganisms could affect intes62 tinal permeability is by altering the expression of claudins. Suzuki and Hara16 discovered that the most common flavonoid present in nature, quercetin, decreases paracellular flux across Caco-2 cells and increases claudin-4 protein expression. Yeh et al.17 determined that chitosan, a widely used food additive, increases paracellular flux in Caco-2 cells. During the postexposure period, when tight-junction function is reestablished, the investigators found that claudin-4 transcription increased. Taken together, the above findings15–17 suggest that claudin activity and expression can be dynamically altered by the diet and by changes in the intestinal microbiota. MILK PROTEIN COMPONENTS AND INTESTINAL CLAUDIN EXPRESSION Recent work by Hering et al.5 adds to the heretofore limited understanding of how nutrition influences claudin biology. The investigators focused on whey, the so-called “fast-digested milk protein,” specifically whey protein concentrate 1 (WPC1). Consistent with earlier studies in both humans and rodents,18,19 these investigators found that WPC1 protected against an interferon gamma (IFNg)-induced tight-junction barrier disturbance. They explored the molecular mechanisms by which whey protein, and WPC1 in particular, exert this effect. Milk, whey protein, and WPC1 all contain high levels of transforming growth factor b1 (TGFb1), which has been reported to improve intestinal tight-junction function.18 To determine if TGFb1 mediates the effects of WPC1 on intestinal tight junctions and to clarify the molecular mechanism by which TGFb1 acts, the authors first showed that WPC1 and TGFb1 both increased transepithelial resistance. To explore the downstream signaling cascade that mediates this effect, the authors next investigated known TGFb1 signaling intermediaries. It had been previously reported that milk and TGFb activated a canonical TGFb reporter construct.18 However, it had also been suggested that Smad-independent pathways mediated the effects of TGFb on epithelial tight junctions.20 As noted, claudin-4 has been previously reported to regulate tight-junction function. Interestingly, these investigators found that WPC1 and TGFb1 increased expression of claudin-4 but did not affect the expression of claudin-1, -2, -5, -7, or occludin. They therefore focused on claudin-4 and used a claudin-4 promoter construct to evaluate cell-signaling pathways entrained by WPC1 and TGFb1. As compared with nonfat dried milk, which has less TGFb1, WPC1 activated the claudin-4 promoter more effectively. TGFb1 was also able to induce this construct. This activation was significantly enhanced by overexpression of Smad-4, a transcription factor known to be a target of TGFb1.21 Deletional analysis, as well as mutaNutrition Reviews® Vol. 71(1):60–65 tion of the Smad-4 binding site of the claudin-4 promoter, established that Smad-4 binding was required for the ability of both TGFb1 and WPC1 to activate the promoter. Thus, HT-29/B6 cells treated with TGFb1 (60 ng/L) for 48 hours showed an increase in the activity of the claudin-4 promoter. Overexpression of Smad-4 augmented this effect, while mutation of the Smad-4 binding site in the claudin-4 promoter abolished the ability of TGFb1 to induce claudin-4 promoter activity. To determine if TGFb1 mediated the effects of WPC1 on claudin-4 transcription, cells were transfected with a claudin-4 promoter and treated with WPC1 in the presence of neutralizing antibodies to TGFb1, 2, and 3. These neutralizing antibodies blocked the stimulatory effect of WPC1 on the promoter. Hering et al.5 concluded that WPC1 mediates its action on intestinal barrier function in part by inducing claudin-4 expression via a TGFb/Smad-4 signaling cascade. These studies provide a plausible molecular mechanism by which milk and whey protein exert their salutary effect on intestinal epithelial integrity. While these models of overexpression support a role for TGFb in Smad-4-mediated regulation of tight junctions, complementary studies involving knockdown of endogenous Smad-4 would provide key additional support for this model. The potential clinical relevance of this proposed molecular pathway is supported by in vivo evidence that TGFb has beneficial effects on the intestinal barrier. In a mouse model, oral administration of 500 mL of cow milk containing TGFb (3,000 ng/L) daily for 2 weeks before induction of colitis and endotoxemia ameliorated both tissue damage and mortality.18 In the neonate, TGFb has been found to be important for gut homeostasis and regulation of inflamation.22 Hering et al.5 suggest that TGFb1 in concentrations as little as 60 ng/L can be beneficial to mucosal restitution. The WPC1 used by Hering et al.5 contained 30–60 ng/g of TGFb1.Although the dilutional effect of gastric and intestinal secretions on ingested WPC1 is not known, it seems reasonable to assume that the ingestion of several grams of WPC1 would result in luminal TGFb1 concentrations in the 60 ng/L range. It is plausible that ingestion of WPC1 could induce biologically significant effects on intestinal barrier protection. Thus, TGFb1-rich WPC1 may have clinical efficacy in gastrointestinal disorders characterized by barrier dysfunction. This is especially appealing in the neonate, in whom the dilution factor between the mouth and duodenum is less than in the adult. In addition to whey protein, casein, the most abundant protein in milk, may influence barrier function and claudins. Visser et al.23 reported that a high-casein diet improved intestinal barrier function in diabetes-prone BioBreeding (DP-BB) rats. At weaning, DP-BB rats were Nutrition Reviews® Vol. 71(1):60–65 placed on a high-casein (HC) diet (containing 200 g/kg casein) or a standard plant-based diet for 65 days. Rats on the HC diet had improved intestinal barrier function as assessed by measuring the ratio of lactulose to mannitol in the urine after oral administration of lactulose and mannitol.23 Serum zonulin levels, which correlate strongly with intestinal permeability, were lower in the animals on the HC diet.23 The HC animals also had increased transepithelial resistance as compared with animals on the standard diet. Quantitative polymerase chain reaction was performed using RNA isolated from the ileum of the HC animals to investigate the effect of the diet on expression of tight-junction proteins. Rats on the HC diet had increased expression of pore-sealing claudin-1 and a reduced expression of pore-forming claudin-2.23 Interestingly, Hering et al.5 found that caseinbased diets contain little TGFb1. These data suggest that the HC diet reduces intestinal permeability in this experimental rat model by a TGFb1-independent mechanism. While the anti-inflammatory effects of TGFb within the intestine have long been known24,25 and are mediated, in part, by modulation of the relative proportions of proand anti-inflammatory CD4+ T cell subsets, these data support an alternative mechanism. The studies of Hering et al.5 and Visser et al.23 are consistent with the idea that milk protein components improve intestinal barrier function, in part, by altering the expression and functions of claudins via both TGFb1-dependent and TGFb1independent pathways. In support of this hypothesis, previous reports demonstrate that a variety of intestinal diseases characterized by altered permeability are accompanied by changes in claudin expression.26,27 ALTERED CLAUDIN EXPRESSION IN INTESTINAL DISEASE As many as 1.4 million Americans suffer from inflammatory bowel disease.28 Altered intestinal barrier function is a common feature of the major inflammatory bowel diseases, Crohn’s disease and ulcerative colitis, as well as diseases such as collagenous colitis. Increased antigen uptake into the circulation can initiate and intensify epithelial inflammation, while water uptake from the circulation to the intestinal lumen can result in diarrhea. This can lead to an auto-intensifying cycle of disease in which increased permeability results in increased antigen uptake, which further worsens the inflammation and diarrhea. Zeissig et al.27 recently reported changes in claudin expression in colonic biopsies from patients with Crohn’s disease. Expression of pore-sealing claudin-5 and claudin-8 was downregulated, while expression of poreforming claudin-2 was strongly upregulated. Claudin-5 and claudin-8 were also found to redistribute away from 63 the tight junction, as determined by immunohistochemistry. In addition to the changes in the molecular composition of the tight junction, the rate of epithelial cell apoptosis was also found to be upregulated. Alterations in tight-junction structure and function have also been observed in ulcerative colitis. Expression of pore-forming claudin-2 is upregulated, accompanied by an increase in the rate of epithelial cell apoptosis, resulting in epithelial lesions. In collagenous colitis, intestinal barrier regulation is significantly altered, with downregulation of pore-sealing claudin-4 and upregulation of pore-forming claudin-2 observed in some patients.26 Interestingly, and in contrast to the findings in Crohn’s disease and ulcerative colitis, rates of epithelial cell apoptosis were not increased in collagenous colitis. Since downregulation of claudin expression has been reported to occur frequently in inflammatory bowel disease and since TGFb upregulates expression of at least claudin-4, it could be postulated that TGFb might serve as a potential therapeutic agent in inflammatory bowel disease, in part by helping to reestablish intestinal barrier function via its effect on claudins. Consistent with that notion, Fell et al.29,30 have reported that a polymeric diet enriched in TGFb induced healing in pediatric patients with Crohn’s disease. CONCLUSION Tight junctions, including the claudin proteins, are a crucial component of the intestinal barrier responsible for integrity and permeability. The varied effects of claudins on permeability are, for the most part, poorly understood. It has been suggested that claudin function, and thereby tight-junction permeability, can be dynamically altered by the diet and changes in the intestinal microbiota.15–17 Specifically, Hering et al.5 and Visser et al.23 suggest milk protein components improve intestinal barrier function, in part, by altering the expression and functions of claudins. These studies5,23 are limited by the fact that animal models exclusively were studied, leaving uncertain the relevance of these findings in humans. The reliance on overexpression studies to establish the role of Smad-4 in the TGFb1/claudin-4 pathway by Hering et al.5 requires that additional studies be performed before this signaling cascade can be accepted as broadly applicable in vitro and in vivo. Nonetheless, the studies summarized above5,18–23 add to the current understanding of how nutrition influences barrier regulation. Several mechanisms contribute to intestinal barrier dysfunction and disease. Investigation of tight-junction protein regulation of intestinal disease may provide new targets for therapeutic intervention. Further research is necessary to confirm the mechanisms by which milk protein components improve intestinal 64 integrity, as well as whether milk protein components should be considered as a therapy for diseases characterized by intestinal barrier compromise. Clinical trials using whey-protein isolates in humans with intestinal barrier dysfunction would be of considerable interest. In addition to noninvasive assessment of intestinal barrier function, intestinal mucosal biopsies to assess claudin expression would be of interest. If well-designed and properly controlled, such studies would significantly aid in determining whether claudins deserve attention as potential therapeutic targets, and, if so, which ones should be pursued. Acknowledgment All listed authors contributed significantly to the work’s conception, design, data collection, or data interpretation and analysis; participated in the writing or critical revision of the article in a manner sufficient to establish ownership of the intellectual content; and read and approved the version of the manuscript being submitted. Funding. This publication was made possible in part by grant number 460712 from the University of Connecticut (to BMK). Its content is solely the responsibility of the authors and does not necessarily represent the official view of the University of Connecticut. This work was also supported by a grant from the United States Department of Agriculture (USDA 2009-65200-04920, to KLI and JEK). Declaration of interest. The authors have no potential conflicts of interest to declare. REFERENCES 1. Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61:431–437. 2. Ulluwishewa D, Anderson RC, McNabb WC, et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. 3. Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–F876. 4. Overgaard CE, Daugherty BL, Mitchell LA, et al. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid Redox Signal. 2011;15:1791–1193. 5. Hering NA, Andres S, Fromm A, et al. Transforming growth factor-beta, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr. 2011;141:783–789. 6. Furuse M, Fujita K, Hiiragi T, et al. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. 7. Wen H, Watry DD, Marcondes MC, et al. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–8417. 8. Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. 9. Escudero-Esparza A, Jiang WG, Martin TA. The Claudin family and its role in cancer and metastasis. Front Biosci. 2011;16:1069–1083. 10. Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. Nutrition Reviews® Vol. 71(1):60–65 11. Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. 12. Fujita H, Sugimoto K, Inatomi S, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. 13. Tatum R, Zhang Y, Salleng K, et al. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol. 2010;298:F24–F34. 14. Tamura A, Kitano Y, Hata M, et al. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523–534. 15. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–G1034. 16. Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965–974. 17. Yeh TH, Hsu LW, Tseng MT, et al. Mechanism and consequence of chitosanmediated reversible epithelial tight junction opening. Biomaterials. 2011;32: 6164–6173. 18. Ozawa T, Miyata M, Nishimura M, et al. Transforming growth factor-beta activity in commercially available pasteurized cow milk provides protection against inflammation in mice. J Nutr. 2009;139:69–75. 19. Sprong RC, Schonewille AJ, van der Meer R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: role of mucin and microbiota. J Dairy Sci. 2010;93:1364–1371. 20. Howe KL, Reardon C, Wang A, et al. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol. 2005;167:1587–1597. Nutrition Reviews® Vol. 71(1):60–65 21. Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. 22. Penttila IA. Milk-derived transforming growth factor-beta and the infant immune response. J Pediatr. 2010;156(Suppl):S21–S25. 23. Visser JT, Lammers K, Hoogendijk A, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010;53:2621–2628. 24. Kriegel MA, Li MO, Sanjabi S, et al. Transforming growth factor-beta: recent advances on its role in immune tolerance. Curr Rheumatol Rep. 2006;8:138–144. 25. Mantel PY, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol. 2011;677:303– 338. 26. Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23: 379–383. 27. Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. 28. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504– 1517. 29. Fell JM. Control of systemic and local inflammation with transforming growth factor beta containing formulas. JPEN J Parenter Enteral Nutr. 2005; 29(Suppl):S126–S128; discussion S129–S133, S184–S188. 30. Fell JM, Paintin M, Arnaud-Battandier F, et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281–289. 65