* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Human NF-IL6 beta Gene Is Up-Regulated by the EGF Through p38
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
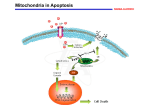
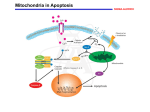
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Dephosphorylation of c-Jun C-terminus is involved in phorbol ester-induced interaction between c-Jun and Sp1 in promoter activation of human 12(S)lipoxygenase Ben-Kuen Chen, Chi-Chen Huang, Wei-Chiao Chang and Wen-Chang Chang Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan c-Jun/Sp1 interaction is essential for growth factor- and phorbol ester-induced expression of genes, including human 12(S)-lipoxygenase. This study examined the underlying mechanism of regulating the interaction between c-Jun and Sp1 upon phorbol 12-myristate 13-acetate (PMA) treatment of human epidermoid carcinoma A431 cells. PMA-induced promoter and enzymatic activities of 12(S)-lipoxygenase were dose-dependently inhibited by a specific calcineurin (PP2B) inhibitor, cyclosporin A. Overexpression of PP2B also enhanced the promoter activity of the 12(S)-lipoxygenase gene in a dose-dependent manner. Moreover, in PMA-treated cells, cyclosporin A inhibited the interaction between c-Jun and Sp1. Overexpression of TAM-67, an N-terminally truncated c-Jun, inhibited the PMA-induced promoter activity of 12(S)-lipoxygenase. TAM-67-M3A, which contains three substitutive alanine at Thr-231, Ser-243, and Ser-249, was about twice as efficacious as TAM-67 in inhibiting the PMA-induced promoter activity of the 12(S)-lipoxygenase gene. GST pull-down assay indicated that, compared to phosphorylated GST-TAM-67 by casein kinase II, GST-TAM-67-M3A bound more efficaciously with Sp1. Taken together, these results indicate that the dephosphorylation of Thr-231, Ser-243, and Ser-249 at the c-Jun C-terminus is essential for the protein-protein interaction between c-Jun and Sp1. Human NF-IL6 beta gene is up-regulated by the EGF through p38 and CREB dependent pathway in A431 cells Ju-Ming Wang and Wen-Chang Chang Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan NF-IL6 beta (also as human C/EBP delta), a member of the leucine zipper transcription factor family, is expressed at higher levels in the lung than in any other tissues. The epidermal growth factor (EGF) is well known to activate MAPKs pathway in many cell lines. In this study, we show the transcriptional activity of NF-IL6 beta is increased by EGF in A431cells. We identified a 349-bp promoter region which was sufficient for basal and EGF-induced transcription of NF-IL6 beta gene. The region contains binding sites for the cAMP response element-binding protein and Sp1 transcription factor. Reporter assays showed the CRE and Sp1 binding sites were important for the EGF inducibility of NF-IL6 beta gene. The p38 kinase signaling pathway was also involved in EGF activation of NF-IL6 beta gene transcription. By pretreatment of the cells with p38 inhibitor SB203580, the EGF-induced NF-IL6 beta gene transcription activity was blocked. Lastly, we found that CREB bound with CRE element of the NF-IL6 beta promoter and played a function role in EGF activation of the NF-IL6 beta reporter gene. Taken together, our results suggested that the activated p38 kinase works through CREB to activate NF-IL6 beta gene expression under EGF treatment. Molecular mechanism of interaction between vitamin D receptor and Sp1 in gene regulation Yu-Chun Huang, Jing-Yi Chen, Lea-Yea Chuang and Wen-Chun Hung Graduate Institute of Medicine, Department of Biochemistry and School of Technology for Medical Sciences, Kaohsiung Medical University, Kaohsiung, Taiwan Vitamin D3 has been shown to up-regulate p27Kip1 expression via Sp1 and NF-Y binding sites in the p27Kip1 promoter. However, whether vitamin D3 receptor (VDR) involves in this process is unclear. In this study, we demonstrated that expression of VDR in SW620 cells, which exhibited low level of endogenous VDR, increased vitamin D3-stimulated p27Kip1 promoter activity. On the contrary, suppression of Sp1 expression by small interference RNA (siRNA) reduced the stimulation of p27 Kip1 promoter activity by vitamin D3 in LNCaP cells. DNA affinity precipitation assay (DAPA) and chromatin immunoprecipitation (CHIP) assay showed that VDR bound to the p27Kip1 promoter in vitro and in vivo. In addition, we also demonstrated that VDR interacted with Sp1 in vitro and in cells. Collectively, our results suggest that VDR is involved in the induction of p27Kip1 by vitamin D3 and may interact with Sp1 to modulate the expression of target genes which lack VDR response element (VDRE) in their promoters. We next extend our finding and try to answer the molecular mechanism by which the VDR/Sp1 complex regulates gene expression. By using the double strand DNA probe corresponding to the region of the human p27 Kip1 promoter to precipitate the interacting proteins, we found that VDR indirectly bound to the DNA probe via Sp1. Additionally, we found that some VDR coactivators including SRC-1 and TRAP 250 were also co-precipitated by the DNA probe. These results suggest that activation of p27Kip1 expression by the VDR/Sp1 complex may be mediated via the activation domain of VDR, rather than that of Sp1. Indeed, deletion of the AF2 domain of VDR abolished the interaction between VDR and their coactivators and reduced the activation of p27Kip1 promoter activity by the VDR/Sp1 complex. Taken together, our results suggest that Sp1 functions as a carrier protein to bring VDR to the Sp1 binding site in the p27Kip1 promoter and VDR then recruits the co-activators via its activation domain to stimulate p27Kip1 gene expression. C/EBPs and Sp1 mediate induction of rat Mrp3 gene Shwu-Jen Tzeng and Jin-ding Huang Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan Mrp3 protein expression in liver is induced under conditions of cholestasis. In addition to cholestasis, Mrp3 expression in rat liver can be induced by treatment with cyp2B inducers, such as diallyl sulfide (DAS), and trans-stilbene oxide (TSO). The goal of this study was to determine 1) whether treatment with DAS and TSO can up-regulated Mrp3 expression in H4IIE cells, and 2) the induction mechanism of Mrp3 expression by DAS and TSO. In H4IIE cells, after DAS or TSO treatment, the Mrp3 expression was dose-dependently induced as shown in RT-PCR. Functional analysis demonstrated that the response elements localizes within the proximal region of Mrp3 promoter. EMSA and supershift assays demonstrated specific binding of Sp1, Sp3, C/EBPα, β, and δ to the region. Transient transfections and luciferase assays revealed that C/EBPα or δ is a stronger transcriptional activator than C/EBPβ. Combined the expression of C/EBP proteins (α, β, δ) and TSO treatment in these cells resulted in a strong synergistic transactivation of this promoter, but dominant negative A-C/EBP (only have DNA binding domain) reduced this effect. The CBP could collaborate with C/EBPs in the activation of Mrp3 promoter. TSO-induced promoter activation was blocked by the viral E1A oncoprotein. These data indicate that the response element of Mrp3 expression by TSO was through the C/EBP and Sp1 sites. The synergistic transactivation of Mrp3 promoter with TSO was mediated by transactivation domain of C/EBP proteins to recruit CBP. The involvement of macrophage stimulating protein/RON signaling pathway in the tumorigenesis of human bladder Yen-Jou Lin, Nan-Haw Chow and Hsiao-Sheng Liu Departments of Microbiology and Immunology, Department of Pathology, and Institute of Molecular Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan RON is a transmembrane receptor tyrosine kinase. The ligand of RON is macrophage-stimulating protein (MSP). The interaction between MSP and RON mediates a number of biological properties, including cell motility and proliferation. In this study, we revealed the possible MSP/RON related signaling pathways and biological activities in the progression of bladder carcinogenesis. A total of nine bladder cancer and one immortalized uroepithelial cell line were employed. Expression of wild-type RON protein was widely detected in many baldder cancer cell lines (such as RT4, TSGH8301, TCCSUP, and UB37), and clinically, 42.9 % and 33% RON expression were detected in invasive bladder cancer and upper urinary tract transitional cell carcinoma, respectively, indicating a significant association with non-papillary growth and multiple tumors of bladder cancer. The bladder cancer TSGH8301 cells showed a MSP-dependent RON phosphorylation. Another bladder cancer UB37 cells exhibited an autocrine MSP/RON relationship. All together, MSP/RON may transduce its signals through either an autocrine or a paracrine manner. The bladder cancer UB47 cells were proven to have a 147bp deletion of RON at the extracelluar domain near transmembrane region. This aberrant RON protein accumulated in the cytoplasm, and exhibited a MSP-independent phosphorylation. However, no mutation in the kinase domain of many bladder cancer specimens was detected by SSCP. Regarding RON related biological effect, overexpression of RON was able to enhance cell growth, migration, and anti-apoptosis. By using PI3K specific inhibitor (Wortmannin) and NFB luciferase report system, we confirmed that PI3K-Akt pathway and NFB were involved in RON-related anti-apoptosis. We also observed a MSP-independent nuclear localization of RON protein. In addition, aberrant localization of RON protein in cytoplasm of the cancer cells was detected in about 3% of the bladder and upper tract cancer samples. All together, we are the first to detect that either wild type or mutant form of RON could translocate into nucleus. Multifunctional mechanisms for RON activation seem to be involved in the progression of bladder cancer. The understanding of RON may provide a molecular basis in developing gene-targeting therapy for human bladder cancer. The participation of p97Eps8 in the growth of cancer cells Tzeng-Horng Leu1, Hsu Hua Yeh2, Ching-Chung Huang1, Ya-Chun Chuang1, Shu Li Su2 Chia-Yin Hsieh1 and Ming-Chei Maa2 1 2 Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan. Institute of Biochemistry, Chung Shan Medical University, Taichung, Taiwan. Our studies on Eps8 expression in v-Src transformed cell indicates that both protein and mRNA level of the 97-kDa and especially the 68-kDa proteins are highly overexpressed. Furthermore, when p97Eps8 is overexpressed in normal C3H10T1/2 fibroblast, it enables focus formation in vitro and tumor formation in mouse. These results suggest that Eps8 might play an important role in v-Src-mediated transformation. To further substantiate this hypothesis, we have studied the anticancer activity of trichostatin A, a specific histone deacetylase inhibitor, in v-Src transformed cells (IV5). We observed that trichostatin A, could effectively inhibit the growth of IV5 and abrogate their ability to form colonies in soft agar. Further analysis demonstrated that while trichostatin A reduced the expression of Eps8, in a dose- and time-dependent manner, both the protein expression and the kinase activity of v-Src remained constant and the abundance as well as the phosphotyrosine level of Src substrates including cortactin, FAK, p130Cas, paxillin, and Shc were not altered. Northern and reverse transcription-polymerase chain reaction analysis revealed the significant reduction of eps8 transcripts in trichoststin A-treated IV5 relative to control. When active Src expressing chicken embryonic cells were forced to overexpress p97Eps8, they became resistant to trichostatin A-mediated antiproliferation. Furthermore, using small interference RNA of eps8, we demonstrated the requirement of Eps8 in the proliferation of IV5. To extend this observation, we have generated eps8 siRNA-transfected SW620 cell line and observed that reduced p97Eps8 expression by eps8 siRNA retard the growth rate of SW620. Thus, our results highlighted a critical role for p97Eps8 in the cell growth of human colorectal cancer cells. Autocrine IL-6 induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion Shuan-Heng Yeh, Shu-Wen Hsiao, I-Hwi Tu, Wu-Wei Lai, Hsiao-Sheng Liu, Ming-Derg Lai and Wu-Chou Su Institude of Basic Medical Scicence, Biomedical Science, and Microbiology, and Department of Surgery and Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan Malignant pleural effusion (MPE) is a poor prognostic sign for patients with non-small cell lung cancer (NSCLC). The generation of MPE is largely regulated by vascular endothelial growth factor (VEGF), and the expression of VEGF is up-regulated by Stat3 in several tumor cells. In this study, we have demonstrated higher expression of IL-6 and VEGF in the pleural fluids of patients with lung adenocarcinoma than those with congestive heart failure. Furthermore, constitutively activated Stat3 was found in tumor cells infiltrated in the pleurae of patients with lung adenocarcinoma and MPE, immunohistochemically. The observations suggested IL-6/Jak2/Stat3 axis is activated in lung adenocarcinoma, which may contribute to VEGF expression. We found higher expression of VEGF (mRNA and protein) in Stat3-positive human lung adenocarcinoma cell-- PC14PE6/AS2 than in Stat3-negative PC14PE6/AS2/dnStat3 cell. In animal model, all the five nude mice injected with PC14P6/AS2 cells developed malignant ascites at 27th day, but none from those injected with PC14P6/AS2/dnStat3 cells. Thirty-one days after injection of neoplastic cells into tail veins, widely metastatic lung lesions with malignant pleural effusion were found in mice received PC14P6/AS2 cells but not in those injected with PC14P6/AS2/dnStat3 cells. The expression of VEGF was also higher in PC14PE6/AS2 induced tumors. Besides possessing higher potential for metastasis, the PC14PE6/AS2 cell is more resistant to anticancer agents-- paclitaxel and cisplatin than is the PC14PE6/AS2/dnStat3 cell. In preliminary proteomic studies, by comparing protein expression patterns of three smoking male with five non-smoking female lung cancer patients, we have identified three proteins overexpressed in non-smoking females. They included α-1 inhibitor III precursor, apolipoprotein E, coatomer protein complex, subunitβ. RNA specimens from cancer cells in MPE and a normal lung cell line (Bes2B) were extracted and sent for Affymatrix oligonucleotide microarray analyses. Under non-supervised clustering analyses, female non-smokers and male smokers were subdivided into two groups. These preliminary results are encouraging and we will do further studies to identify novel genes or proteins those are related to the pathogenesis of the particular disease. ER stress signaling role of pre-S mutants in HBV-related hepatocarcinogenesis Hui-Ching Wang and Ih-Jen Su Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan In our laboratory, we have previously identified two HBV pre-S mutants, and both of which were associated with the disease progression to hepatocellular carcinoma. The expression of both pre-S mutants on Huh7 cells leads to the accumulation of pre-S mutant proteins in ER which in turn activates ER stress signals. We therefore suggested that HBV pre-S mutant protein might confer growth advantages through the activation of ER stress signals. In our study, we first analyzed the potential of gene transactivation by HBV pre-S mutant proteins. Our result shows that HBV pre-S mutant protein can act as a gene transactivator as demonstrated by the PathDetect assay, and can activate reporter genes including NF-B, AP-1, CRE, SRE, p53, NF-AT, and ISRE. Interestingly, most of the signals we have tested were stronger in cells expressing wild type protein than that expressing pre-S2 mutant protein, except the p53 activation. Moreover, it has been demonstrated that the localization in ER of HBV surface proteins might be associated with MAP kinase activation. We next demonstrated that MAP kinase (ERK1/2) can be activated by HBV surface proteins, especially the pre-S2 mutant. With the application of MAP Kinase inhibitor and PKC inhibitor, we next demonstrated that the activation of NF-B by the pre-S mutant protein was mediated by PKC and through the activation of MAP kinase ERK1/2. How the ER associated pre-S mutant proteins coordinate with protein kinase C remains a question to be solved. However, our study shows the possibility of an unfolded protein driven by an abnormal cellular gene or a viral pathogen could induce a signal cascade affecting intracellular biological functions. ER stress signals therefore no longer can be taken just as a protective mechanism against stress condition, it might be a common mechanism used by oncogenic virus in cellular carcinogenesis. Novel signaling upon contact with tumors to suppress the Fas-mediated death signal in T cells Chung-Chen Su, Woei-Jer Chuang, Yan-Shen Shan and Bei-Chang Yang Institute of Basic Medical Sciences and Department of Microbiology and Immnology, College of Medicine, National Cheng Kung University, Tainan, Taiwan Fas/Fas-L signaling system, one of the major apoptosis-mediating pathways, plays an important role in various immune functions such as cytotoxicity, immune homeostasis, and self-tolerance. There are pieces of evidence showing that Fas-L contributes to tumor escape from immune surveillance by killing tumor infiltrating cells, which is known as the Fas-counterattack mechanism. However, we have recently found that Fas signaling can also induce gene expression such as IL-10 in T cells without leading to cell death. In addition, we found that most of tumor-infiltrating cells isolated from gastric carcinoma were not apoptotic despite of enhanced caspase 3 activity. To evaluate the apoptosis in T cells in contact with tumor cells, we used an in vitro coculture system in this study. Apoptosis was measured by annexin V stain detecting altered membrane asymmetry and propidium iodine stain detecting subG0 cell population. Treatment of Fas-agonistic antibody, CH-11, led to caspase 3 activation in Jurkat cells through mitochondria-dependent and -independent pathways leading finally to CAD activation to cut off DNA. When Jurkat cells were in direct cell contact with glioma cells, they were less susceptible to the CH-11 induced apoptosis as revealed by decrease in caspase-3 activation, amelioration of loss of mitochondria membrane potential, and reduction in breakage of DNA as compared to Jurkat cells alone in culture. Tumor cell lines such as HeLa, A549, Huh-7 protected Jurkat cells from Fas-mediated apoptosis as glioma did. No new protein synthesis was required for the protective effect of cell-cell contact for Jurkat cells. Protein kinase inhibitors including SP600125, PD98059, and SB202190 did not affect on this protection. RGD-containing peptide could partially block this tumor cell-contact associated protection. Together, our results indicate that direct contact with tumor cells may trigger a novel protective signaling in Jurkat cells to suppress Fas-mediated apoptosis. Caspase-3 and p21WAF1 expressions in human cervical cancer, and novel mechanisms underlying GnRH-agonist induced shrinkage of uterine leiomyoma Chao-Po Lin, Meng-Ru Shen, Soon-Cen Huang, Ming-Jer Tang and Cheng-Yang Chou Institute of Clinical Medicine and Departments of Pharmacology, Physiology, and Obstetrics & Gynecology, College of Medicine, National Cheng Kung University, Tainan, Taiwan Our previous results showed that procaspase-3 and p21WAF1 were overexpressed in human cervical cancer and in benign uterine leiomyoma. Activation of caspase 3 with subsequent cleavage of p21WAF1 was found in some of the cervical cancer specimens. In contrast, cleavage/activation of overexpressed caspase 3 is absent in leiomyoma, suggesting that caspase-3 and p21WAF1 may play differential roles in these two tumors. In this study, we have examined the association of p21WAF1 expression with cytokine STAT1 expression, and to correlate with survival of cervical cancer patients. Meanwhile, we have utilized samples from leiomyoma patients undergoing GnRH agonist therapy to elucidate the mechanisms underlying cell death and tumor shrinkage following GnRH agonist therapy. Among the 64 cervical cancer patients studied, patients with adenocarcinoma (n=12) exhibited much less caspase 3, p21WAF1, and STAT1 as compared with patients with squamous cell carcinoma. Of the 52 patients with squamous cell carcinoma, increased expression of caspase 3 and p21WAF1 is associated with the expression of STAT1 and favorable disease survival. Furthermore, the p21WAF1 expression was associated with cell apoptosis. In uterine leiomyoma, the fluctuation of FasL and caspase 3 expressions do not accompany changes of apoptosis. The absence of anticipated increase in caspases 6, 8, 9, and 10 further confirms that neither Fas/FasL nor cytochrome c-related apoptosis pathways have been involved. In the uterine smooth muscle cells, Fas and FasL predominantly exist in the cytoplasm instead of on cell membrane. These findings account for the relative resistance of leiomyomas to apoptosis. In addition, GnRH agonist treatment induces overexpression of poly(ADP-ribose) polymerase (PARP), and the accumulation of its end product poly(ADP-ribose) (PAR). This is correlated with the clinical response of GnRH agonist therapy that GnRH agonist induces more leiomyoma degeneration. Power Doppler sonography indicates a progressive decrease in blood supply to tumor following GnRH agonist treatment. In vitro experiments exhibit that the deprivation of serum or ovarian hormones or GnRH agonist directly fails to induce PARP and PAR production. Our results suggest that reduced blood flow and subsequent ischemic damages in leiomyoma could be responsible for PARP overexpression, and tumor degeneration caused by GnRH agonist treatment. Substratum rigidity controls epithelial cell life and death Wen-Tai Chiu*, Yao-Hsien Wang*, Yang-Kao Wang and Ming-Jer Tang Department of Physiology and Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan In order to delineate how the substratum rigidity controlled cell life and death, we employed cell cultures on type I collagen gel. In this study, we established that collagen gel induced apoptosis of epithelial (NMuMG, BS-C-1, LLC-PK1, NRK-52E and BAEC) but not mesenchymal (293 and NIH-3T3) or tumor (HK-2, U-373MG, OC-2, DOK, SSC-25, HSC-3 and Chang Liver) cell lines. Biochemical and morphological studies all indicated that collagen gel-induced epithelial cell apoptosis was mediated by the physical property, i.e. substratum rigidity, of the gel. To delineate whether rigidity controlled epithelial cell apoptosis, we employed collagen gels that were constituted of fixed biochemical composition but exhibited different rigidity due to cross-linking or physical disruption of collagen fibrils. We found that collagen gel prepared from older rat ameliorated contracted morphology and apoptosis. On the other hand, a reduction in rigidity by sonication of collagen fibrils augmented collagen gel-induced apoptosis. As assessed by rheometry and dynamic mechanical analyzer, the rigidity of collagen gel ranges from 10 to 120 Pa. Furthermore, low rigidity-induced apoptosis signal is mediated neither by 21 integrin nor discoidin domain receptor. In addition, it is not mediated by mitochondria release of cytochrome c and could not be prevented by Bcl-2 overexpression. In general, it is partially mediated by a caspase-dependent pathway. Taken together, this study establishes that rigidity of collagen gel controls epithelial cell life and death, and the signal mechanism underlying which remains to be elucidated. Lithium blocks ceramide-induced T cell apoptosis by inhibiting protein phosphatase 2A and initiator caspases activation Chia-Ling Chen, Chiou-Feng Lin, Ming-Shiou Jan, Li-Jin Hsu and Yee-Shin Lin Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan Lithium, a neuroprotective drug used for bipolar disorder, has been shown to confer protection against cytotoxicity. Although the anti-apoptotic effect of lithium has been proposed, the molecular mechanisms by which lithium inhibits cell apoptosis remain unclear. Our studies in ceramide-induced T cell apoptosis showed that lithium inhibited the death events through the blockage on the reduction of mitochondrial transmembrane potential (MMP), cytochrome c release, and caspase-9 and -3 activation. Ceramide-induced sequential caspase-2 and -8 activation before mitochondrial dysfunction was demonstrated. How caspase-2 regulates caspase-8 activation is still unknown. The mechanisms of caspase-2 activation are also unclear. Interestingly, the activation of initiator caspases by ceramide was also eliminated by lithium. Using specific protein phosphatase 2A (PP2A) inhibitor okadaic acid, the inhibition on ceramide-induced apoptosis and MMP reduction was shown. Moreover, okadaic acid reduced the mitochondrial intrinsic pathways of apoptosis by inactivating initiator caspases similar to that of lithium. Recent study showed that Bcl-2 dysfunction caused by ceramide was facilitated through the PP2A-regulated pathway. Roles that lithium may play in PP2A-mediated Bcl-2 dysfunction and initiator caspases activation are currently investigated. We speculate that the anti-apoptotic role of lithium against ceramide-induced cell apoptosis may be mediated by caspase-2 inactivation through the inhibition of PP2A activity.