* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mihaela_Leonida_Abstract
Immunoprecipitation wikipedia , lookup
Structural alignment wikipedia , lookup
Rosetta@home wikipedia , lookup
List of types of proteins wikipedia , lookup
Homology modeling wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein design wikipedia , lookup
Protein domain wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein folding wikipedia , lookup
Protein structure prediction wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
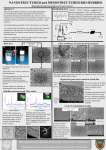
Bioactive proteins encapsulated in nanoparticulate chitosan from different sources Mihaela D. Leonida School of Natural Sciences, Fairleigh Dickinson University, Teaneck, NJ, USA [email protected], http://view2.fdu.edu/faculty-staff-profile-pages/mihaela_leonida/ Although the use of proteins and peptides as therapeutic agents and as antimicrobials in the food industry has expanded, problems are still encountered due to their susceptibility to proteolytic degradation, low solubility, and often physicochemical instability. As a solution to these problems, we proposed encapsulation in a delivery system based on chitosan nanoparticles for protection and slow release. Chitosan nanoparticles have been widely studied as prospective drug carriers for therapeutic proteins and genes due to their remarkable properties of biodegradability, biocompatibility, antimicrobial activity, and inexpensive and mild preparation method [1]. This study seeks to further explore the encapsulation efficiency of proteins in chitosan nanoparticles and subsequent protein release kinetics as a function of the loaded protein concentration. A secondary goal is assessing the feasibility of using chitosan nanoparticles as a candidate vector for nisin in food products and histaminase in applications to the skin. Nisin is a natural, toxicologically safe and effective food preservative [2] which was used as target protein for this study. Bovine serum albumin (BSA) was used as model protein. Nanoparticulate chitosan was prepared through ionotropic gelation, using sodium tripolyphosphate as crosslinker. Protein, in different ratios, was loaded via the incorporation method. The resulting nanoparticles were characterized by IR spectroscopy, zeta potential, and colloidal titration. The encapsulation efficiency (EE) and loading capacity (LC) were determined as well using UV spectroscopy. All protein-loaded nanoparticles showed cross-linking, their IR spectra resembling the one of nanochitosan alone. Both BSA and nisin demonstrated relatively high encapsulation efficiency with BSA demonstrating decrease in EE with increasing protein concentration in particles and nisin presenting a somewhat reversed pattern. LC increased with increasing protein concentration in the preparative mixtures. Particles with low protein concentration have demonstrated initial burst release followed by a slower and constant release pattern, while particles with protein concentrations of 0.5 mg/ml and 1.0 mg/ml have shown controlled sustained protein release without any burst peaks. A subsequent set of preparations investigated the encapsulation of diamine oxidase (histaminase, DAO) in nanoparticulate chitosan using ionic gelation as well with sodium tripolyphosphate as crosslinker. DAO was chosen due to its multifaceted physiological involvement: in wound healing, in detoxification, in cell growth by regulating the intracellular di- and polyamine levels, and the aldehyde products of its reaction might have a key role in the biosynthesis of some alkaloids [3]. Chitosans prepared from two different natural sources were used in the preparations (shrimp and fungal) and the nanocomposites were characterized. Our results show that using chitosan nanoparticles as a time-release protein carrier system has promise for applications such as a nisin carrier in the food industry or as a DAO delivery system in applications to the skin. [1] M.D. Leonida and I. Kumar, Bionanomaterials for skin regeneration (Springer-Nature), 69 (2016) [2] J. Delves-Broughton, Food Technology 44, 100 (1990). [3] G. Floris and A. Finazzi Agro , Bioenergetics 87 (2013).