* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download A PEST-like Sequence in the N-Terminal Cytoplasmic Domain of
Ultrasensitivity wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Metalloprotein wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Expression vector wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Gene regulatory network wikipedia , lookup
Signal transduction wikipedia , lookup
Biochemistry wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Biochemical cascade wikipedia , lookup
Paracrine signalling wikipedia , lookup
Magnesium transporter wikipedia , lookup
Point mutation wikipedia , lookup
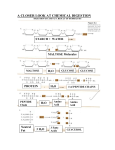
4518 Biochemistry 2000, 39, 4518-4526 A PEST-like Sequence in the N-Terminal Cytoplasmic Domain of Saccharomyces Maltose Permease Is Required for Glucose-Induced Proteolysis and Rapid Inactivation of Transport Activity† Igor Medintz,‡ Xin Wang, Thomas Hradek, and Corinne A. Michels* Biology Department, Queens College and the Graduate School of the City UniVersity of New York, 65-30 Kissena BouleVard, Flushing, New York 11367 ReceiVed October 22, 1999; ReVised Manuscript ReceiVed February 7, 2000 ABSTRACT: Maltose permease is required for maltose transport into Saccharomyces cells. Glucose addition to maltose-fermenting cells causes selective delivery of this integral plasma membrane protein to the yeast vacuole via endocytosis for degradation by resident proteases. This glucose-induced degradation is independent of the proteasome but requires ubiquitin and certain ubiquitin conjugating enzymes. We used mutation analysis to identify target sequences in Mal61/HA maltose permease involved in its selective glucose-induced degradation. A nonsense mutation was introduced at codon 581, creating a truncated functional maltose permease. Additional missense mutations were introduced into the mal61/HA-581NS allele, altering potential phosphorylation and ubiquitination sites. No significant effect was seen on the rate of glucose-induced degradation of these mutant proteins. Deletion mutations were constructed, removing residues 2-30, 31-60, 61-90, and 49-78 of the N-terminal cytoplasmic domain, as well as a missense mutation of a dileucine motif. Results indicate that the proline-, glutamate-, aspartate-, serine-, and threoninerich (PEST) sequence found in the N-terminal cytoplasmic domain, particularly residues 49-78, is required for glucose-induced degradation of Mal61/HAp and for the rapid glucose-induced inactivation of maltose transport activity. The decreased rate of glucose-induced degradation correlates with a decrease in the level of glucose-induced ubiquitination of the ∆PEST mutant permease. In addition, newly synthesized mutant permease proteins lacking residues 49-78 or carrying an alteration in the dileucine motif, residues 69 and 70, are resistant to glucose-induced inactivation of maltose transport activity. This N-terminal PEST-like sequence is the target of both the Rgt2p-dependent and the Glc7p-Reg1p-dependent glucose signaling pathways. Maltose-fermenting Saccharomyces cells require maltose permease to transport maltose across the plasma membrane and for induction of MAL gene expression (1, 2). Addition of glucose to a maltose-fermenting culture causes a biphasic loss of transport activity, characterized by an initial very rapid inactivation of maltose transport activity followed by a slower loss in transport activity that correlates with the degradation of maltose permease protein (3). This degradation is dependent on endocytosis, vesicle sorting, and the vacuolar proteolysis enzymes and is independent of the proteasome (3). As has been shown for a number of yeast and mammalian plasma membrane proteins, we found that ubiquitin and specific components of the ubiquitin conjugating system play an important role in the glucose-induced † This work was supported by grants to C.A.M. from the National Institute of General Medical Sciences (GM28216 and GM49280). T.H. received an undergraduate research stipend from a Howard Hughes Biomedical Institute grant to Queens College. This work was carried out in partial fulfillment of the requirements for the Ph.D. degree from the Graduate School of the City University of New York (I.M. and X.W.). * Corresponding author. Telephone: (718)-997-3410. Fax: (718)997-3431. E-mail: [email protected]. ‡ Current address: Department of Chemistry, University of California at Berkeley, Berkeley, CA 94720. degradation of maltose permease (reviewed in refs 4 and 5). In this report, we use mutation analysis of the N- and C-terminal cytoplasmic domains of maltose permease to identify sequences required for the glucose-induced inactivation and proteolysis of this integral plasma membrane protein. Mutation analysis was used to define the ubiquitination and endocytosis targeting signals in several yeast membrane proteins. These include the SINNDAKSS motif in the C-terminal cytoplasmic domain of the Ste2 R-factor receptor (6, 7). Deletion analysis demonstrated that the middlespanning linker region of the ABC transporter, Ste6p, contains a signal that mediates ubiquitination and rapid turnover of this protein (8, 9). PEST sequences, regions rich in proline, glutamate, aspartate, serine, and threonine, are found in many short-lived proteins. Phosphorylation of serine and threonine residues in PEST sequences is suggested to be associated with regulated degradation, as has been demonstrated for a putative PEST-like sequence in Fur4p, the Saccharomyces uracil permease (10, 11). Other internalization signals have been described in yeast membrane spanning proteins that do not appear to be associated with regulated phosphorylation including the dileucine motif in the general amino acid permease Gap1 and the NPF signal of the a-factor receptor Ste3 (12, 13). 10.1021/bi992455a CCC: $19.00 © 2000 American Chemical Society Published on Web 03/22/2000 Mutation of Maltose Permease Biochemistry, Vol. 39, No. 15, 2000 4519 Table 1: List of Oligonucleotides Used for in Vitro Mutagenesis of MAL61/HAa MAL61/HA allele C-terminal mutations mal61/HA-581NS mal61/HA-581NS(P577A) mal61/HA-581NS(P566A) mal61/HA-581NS(S572A, T573A) mal61/HA-581NS(K569A, K571A,K574A) N-terminal mutations mal61/HA-(∆2-30) mal61/HA-(∆31-60) mal61/HA-(∆61-90) mal61/HA-(∆49-78) mal61/HA-(∆49-60) mal61/HA-(∆61-78) mal61/HA-(L69A,L70A) oligonucleotide sequence (5′-3′) annealing site GCAGCTGCTGCTTTTCAAGCTGCAAAAGG AGCTGCAAAAGCGTCGACTTTAGT CTTTCTTGCTGCAACACCAAG GCAAAAGGGTCGACTTTAGCCGCCTTGAACTTGAACTTTCTTGC 1757-1729 1740-1717 1707-1687 1736-1699 AGGGTCGACTCTAGTCGATCTGAATCTTCTTGCTGGAACACC 1731-1699 TTGCTCCTCCATGTCTATCGAGTTCAAAGGACCACCCAAGCTAG CGTAATCTGG GAGAAGGTCGGGGACTTCTTCATTATTATCTTCGGTAGCGTTCA CGCCATTCTCGATCTC CCAAGCAGCAGCTTTTGGATATGTCTTCAAAGCTGTGTTTGGTAT TAGTGAACCTGGACCGTACTC GAGTGGCATTCCCCTCTCACTTTCATCTGCCTCGGAAAGATCAA AATCACTTTTCTTACCTTGCTCCTCC CATCGAGAAGGTCGGGGACTTCTTCATTATTATCGGAAAGATCA AAATCACTTTTCTTACCTTGCTCCTCC GAGTGGCATTCCCCTCTCACTTTCATCTGCCTCGTTTGGTATTAG TGAACCTGGACCGTACTC GGCGTCCTGCATAGCTTCATCGGCAGCGTCGGGGACTTCTTC 117-91, HA tag 210-181, 90-61 306-271, 180-151 267-235, 145-110 214-183, 145-110 276-235, 180-151 231-191 The nucleotide numbers are based on the sequence of MAL61 (2) with base +1 as the first base in the MAL61 open reading frame and do not include the HA tag (described in ref 3). The 5′ 26 bases of the oligonucleotide used to create mal61/HA-(∆2-30) are complementary to this HA-tag sequence. Underlines indicate the mutant alterations. a Mal61/HA1 maltose permease is found in both phosphorylated and dephosphorylated forms in maltose-fermenting cells, and our previous studies indicate that the level of phosphorylation increases in the presence of glucose (3). The N-terminal, C-terminal, and central cytoplasmic domains of Mal61/HA maltose permease are rich in serine and threonine and contain several potential phosphorylation sites (see Figure 1) (2). A PEST region spans residues 48-79 of the N-terminal cytoplasmic domain (2). Brondijk et al. (14) investigated the role of several of these sites located in the central cytoplasmic domain of Mal61p and found that serine295, located immediately C-terminal to TMD 6, is essential for glucose-induced proteolysis of maltose permease. Interestingly, the rate of glucose-induced inactivation of maltose transport activity is only slightly decreased in this mutant, indicating that permease internalization is occurring at near normal rates but delivery to the vacuole or proteolysis is compromised. We undertook to define sites in Mal61/HAp involved in the initial steps of internalization. Our results suggest that the N-terminal PEST region mediates glucoseinduced internalization and thereby controls the initiating events for the proteolysis of maltose permease. Deletion of the PEST sequence decreases the level of glucose-induced ubiquitination of Mal61/HA maltose permease. An additional glucose effect on the secretion and/or localization of maltose permease was identified that also requires residues in the PEST region, particularly the dileucine motif. The C-terminal cytoplasmic domain does not appear to be involved in either of these functions. MATERIALS AND METHODS Strain Construction. Strain CMY1001 (genotype MATa MAL61/HA MAL12 MAL13 leu2 ura3-52 lys2-801 ade2-101 trp1-∆63 his3-∆200) contains the MAL61 hemagglutintagged gene construct as described in Medintz et al. (3). It 1 Abbreviation: HA, hemagglutinin epitope. contains a single MAL1 locus, and the maltose permease gene at this MAL1 locus is replaced by the epitope-tagged MAL61/ HA allele derived from the MAL6 maltose permease gene MAL61. A mal61/HA null derivative of CMY1001 was constructed by PCR-based gene disruption as follows (15). The upstream (5′) primer consists of 45 bases of sequence complementary to the upstream region of MAL61/HA, followed by 19 bases of sequence complementary to the HIS3 gene 5′ sequence. Its sequence is 5′GTACTCAGCATATAAAGAGACACAATATACTCCATACTTGTTGTGAGTGGGCTTGGTGAGCGCTAGGAG3′. The downstream (3′) primer contains 45 bases of sequence complementary to sequence 3′ of MAL61/HA, followed by 21 bases 3′ to the HIS3 ORF. The sequence of the downstream primer is 5′CACAACAGATGGGGTGCTTCGCCCTTCATCTACCACAGAAGTTTCCAAATCCACACCGCATAGATCCGTCG3′. These primers were used to amplify the HIS3 gene from plasmid pRS413 (18), and this amplified product was used for a onestep gene replacement of the MAL61/HA sequence present at MAL1 of CMY1001, creating strain CMY1050. Gene replacement was confirmed by Southern analysis. The same process was used to delete the MAL61/HA gene in the end3ts strain CMY1004 (3) to create strain CMY1051 and in the rgt2∆::KANR strain CMY1009 (17) to create strain CMY1052. Plasmid Construction and Mutagenesis. MAL61/HA was constructed from MAL61, encoding maltose permease at the MAL6 locus, by inserting a 12-codon sequence containing the HA epitope tag at the N-terminus of the Mal61 protein (3). MAL61/HA was cloned into the vector pUN30 (16), yielding plasmid pMAL61/HA. This plasmid was used as a template for in vitro mutagenesis using the Bio-Rad MutaGene kit (Bio-Rad, Richland, CA) (19). The mutagenic primers used are listed in Table 1. Alterations in MAL61/ HA were confirmed by sequencing. All mutant alleles were constructed with the full-length MAL61/HA gene as a template except for certain C-terminal cytoplasmic domain 4520 Biochemistry, Vol. 39, No. 15, 2000 Medintz et al. FIGURE 1: Sequence of mutations in the N-terminal and C-terminal cytoplasmic domains of Mal61/HA maltose permease. The rectangular shaded areas represent transmembrane domains. The position of the hemagglutinin epitope tag at the N-terminus is indicated as a circle with the letters HA. Arrows point to altered residues. The residue numbers relate to the sequence of the wild-type Mal61 protein reported by Cheng and Michels (2) and do not include the HA tag. mutations that used the 581-stop mutant allele of MAL61/ HA as a template. Plasmid pRGT2-1, provided by S. Ozcan and M. Johnston, contains the RGT2-1 allele, a constitutive signaling allele of the high-glucose sensor (20, 21). InactiVation Assay Protocol. The inactivation assay protocol is described in detail in Medintz et al. (3). Briefly, strains are grown at 30 °C to very early log phase (OD600 of 0.1-0.3) in maltose-containing selective medium. Cells are harvested and transferred to nitrogen starvation medium (yeast nitrogen base without amino acids and ammonium sulfate) plus 2% glucose (or 3% ethanol as indicated). Cell samples are collected at the indicated times over a 3 h period, and for each sample, maltose transport activity is determined and total cell extracts are prepared for Western analysis. Growth dilution is calculated as the OD600 at time 0 divided by OD600 at time x. All inactivation assays were done at least in duplicate cultures of independent transformants. Transport assays of each sample were done in duplicate, and the standard error was less than 20%. Western Blotting. Western blotting analysis was carried out as described previously (3). The Mal61/HA protein in the extracts is detected using anti-HA-specific antibody and the ECL Western blotting kit (Amersham) or the Vistra-ECF kit (Amersham) and the Storm 860 image analyzer (Molecular Dynamics). The relative protein levels in each sample were determined using the Storm 860 image capture software by densitometric comparison to the zero time sample. Western analysis was done in duplicate on extracts prepared from duplicate experiments carried out with at least two independent transformants. To determine the half-life of the mutant proteins, the data from the Western analysis of several experiments were plotted and linear least-squares analysis was used to determine the line of best fit and the time point at which 50% of the initial Mal61/HA protein remained. Sugar Transport Assays. Maltose transport was measured as the rate of uptake of 1 mM [14C]maltose as described in Cheng and Michels (2) and Medintz et al. (3). Assays were done in duplicate on two to three transformants. RESULTS Mutation Analysis of the Mal61/HA C-Terminal Cytoplasmic Domain. The C-terminal cytoplasmic domain of Mal61 maltose permease contains several potential phosphorylation Mutation of Maltose Permease Biochemistry, Vol. 39, No. 15, 2000 4521 Table 2: Phenotype of Mutations in the C-Terminal Cytoplasmic Domain of Mal61/HA Maltose Permeasea MAL61/HA allele MAL61/HA mal61/HA-581NS mal61/HA-581NS (P577A) mal61/HA-581NS (P566A) mal61/HA-581NS (S572A, T573A) mal61/HA-581NS (K569A,K571A, K574A) vector control relative maltose half-life level of transport of maltose maltose Mal61/HA activity permease fermen- protein (% (nM min-1 protein in tation of wild type) mg-1) glucose (h) + + + 100 91 83 5.96 1.71 1.82 1.7 2 0.5 + 72 1.30 2 + 67 0.71 2.4 + 99 0.95 1.2 - - 0.06 ND a MAL61/HA and the mutant alleles are carried on the CEN vector pUN30 [Elledge and Davis (18)] and were introduced into the maltose permease null strain CMY1050 (mal61∆::HIS3) for analysis of phenotype. The Mal61/HA maltose permease relative protein levels were determined by comparison of Western blots using total cell extracts from cells grown in selective medium lacking tryptophan plus 2% maltose. Maltose transport activity was determined in at least duplicate transformants grown in selective medium lacking tryptophan plus 2% maltose. The half-life of maltose permease was determined as described in Materials and Methods from the results of glucose-induced inactivation assays performed in duplicate on at least duplicate transformants. ND ) not determined. sites and nearby lysine residues (Figure 1). Using sitedirected mutagenesis a nonsense mutation was introduced at codon 581 truncating the C-terminal cytoplasmic domain to approximately 36 residues (see Figure 1A). This mal61/ HA-581NS allele, when introduced into strain CMY1050 carrying a deletion of the chromosomal maltose permease gene, complements the maltose-nonfermenting phenotype. Transformants carrying mal61/HA-581NS are maltose inducible, ferment maltose, and express approximately normal levels of mutant maltose permease protein (determined by Western blot) and maltase activity, but maltose transport activity is approximately 25-35% that of transformants expressing MAL61/HA (Table 2). The glucose-induced halflife of mal61/HA-581NS permease is not significantly different from that of the wild-type Mal61/HAp, 2 h vs 1.7 h. Additional missense mutations were introduced into the mal61/HA-581NS allele, altering residues from 566 to 574 that might affect secondary structure, sites of phosphorylation, or ubiquitination of the truncated C-terminal cytoplasmic domain. These include mal61/HA-581NS(P577A), mal61/ HA-581NS(P566A), mal61/HA-581NS(S572A,T573A), and mal61/HA-581NS(K569A,K571A,K574A). When introduced into CMY1050, all of the mutant maltose permease proteins are expressed at approximately normal levels and transformants are capable of fermentative growth on maltosecontaining media (see Table 2). Maltose transport activity of strains expressing these mutant permeases range from 12% of wild-type levels for mal61/HA-581NS(S572A,T573A) to 30% for mal61/HA-581NS(P577A). The rate of glucoseinduced proteolysis of these mutant permeases is not significantly changed, and in some cases the rate is increased. The mutant permease mal61/HA-581NS(P577A) exhibits a Table 3: Phenotype of Mutations in the N-Terminal Cytoplasmic Domain of Mal61/HA Maltose Permeasea MAL61/HA allele MAL61/HA mal61/HA(∆2-30) mal61/HA(∆31-60) mal61/HA(∆61-90) mal61/HA(∆49-78) mal61/HA(∆49-60) mal61/HA(∆61-78) mal61/HA(L69A,L70A) vector control a maltose relative half-life of transport level of maltose maltose activity Mal61/HA permease fermen- (nM min-1 protein (% protein in tation mg-1) of wild type) glucose (h) + + + + + + + - 5.96 0.71 2.07 0.07 0.43 0.83 0.53 0.53 0.06 100 85 76 34 71 126 81 111 ND 1.7 3.5 >4 >4 >4 3.4 >4 1.5 ND See Table 2 for details. ND ) not determined. glucose-induced half-life of 0.5 h, approximately 3-fold faster than that of the wild-type protein. Mutation Analysis of the Mal61/HA Permease N-Terminal Cytoplasmic Domain. Mal61/HAp contains an N-terminal cytoplasmic PEST sequence that spans residues 49-78, and several additional serine residues flank this site (2). Located within the PEST sequence is a dileucine motif at residues 69 and 70. To investigate residues critical for glucoseinduced inactivation, a series of deletion mutations of this domain along with specific point mutations were constructed. These include deletions of residues 2-30, 31-60, 61-90, 49-78, 49-60, and 61-78 and point mutations of the dileucine motif at residues 69 and 70 to alanine (see Figure 1). Plasmid-borne mutant alleles were transformed into strain CMY1050 and the phenotypes determined (see Table 3). Only mal61/HA(∆61-90) is unable to complement the nonfermenting phenotype of CMY1050. Transformants carrying the other mutant alleles all expressed reduced levels of maltose transport activity, ranging from 7% to 35% of wild type. The relative level of mutant Mal61/HA protein expressed in these cells was determined by Western blots of total cell extracts prepared from transformants grown in synthetic medium with 2% glycerol/3% lactate plus 2% maltose (Table 3). These do not vary more than 25-30% from wild-type expression levels with the exception of Mal61/HA(∆61-90)p. While Mal61/HA(∆61-90) mutant permease lacks significant maltose transport activity, expression of the mutant protein is maltose inducible and about one-third the level of wild type. Figure 2 presents the results of glucose-induced inactivation assays carried out on these N-terminal mutants. Transformants expressing mutant permeases lacking all or a portion of the PEST sequence exhibit dramatically reduced rates of glucose-induced degradation. In transformants expressing mutant permeases with deletions of residues 4978, 49-60, and 61-78, glucose stimulates an immediate increase in maltose transport activity, from 3- to 9-fold. Variation in the level of increase is seen among different transformants but appears to be constant in a particular transformant (data not shown). Lineweaver-Burk kinetic analysis of mal61/HA(∆49-78) maltose transport at time 0 and 1 h after the addition of glucose found that the increased transport activity results from an increase in Vmax of the transporter and not from a change in Km (data not shown). During the 3 h time course of inactivation experiments, the 4522 Biochemistry, Vol. 39, No. 15, 2000 Medintz et al. FIGURE 2: Glucose-induced inactivation of maltose permease mutant alleles with alterations in the N-terminal cytoplasmic domain. CMY1050 transformants carrying either plasmid pMAL61/HA, pMAL61/HA(∆2-30), pMAL61/HA(∆31-60), pMAL61/HA(∆49-78), pMAL61/ HA(∆49-60), pMAL61/HA(∆61-78), pMAL61/HA(∆60-90), or pMAL61/HA(L69A,L70A) were grown on selective medium plus 2% maltose, harvested, and transferred to nitrogen starvation medium plus 2% glucose. At the indicated times the OD600 was determined, and aliquots of the culture were removed for maltose transport assays and the preparation of total protein extracts for Western analysis of Mal61/HA protein levels, as described in Materials and Methods. The Vistra-ECF Western blotting visualization system (Amersham) and the Molecular Dynamics storm image analyzer were used to capture the Western image and quantitate the relative density of each time point. Representative Western blots are shown for strains carrying pMAL61/HA and pMAL61/HA(∆49-78). The quantitation data used in the graphs were obtained from the average of at least two experiments, each run on duplicate gels, and scanned twice. The relative levels of Mal61/HA protein (b) and maltose transport activity (O) compared to the zero time sample are plotted along with the growth dilution (9, dotted line). Growth dilution represents the growth of the culture during the course of the experiment and is calculated as the OD600 at time 0 divided by the OD600 time x. maltose transport activity remains at this elevated level, indicating that internalization of these mutant permeases is blocked. Thus, loss of residues 49-78 appears to provide resistance to internalization. The strain expressing the mal61/HA(L69A,L70A) permease reproducibly exhibits an approximately 50% increase in maltose transport activity about 1 h after glucose addition, but in contrast to the ∆PEST mutations, transport activity drops to 20% of initial levels by the third hour (Figure 2). Glucose-induced degradation of this dileucine motif mutant permease protein appears to be normal. Mutation of the dileucine motif provides resistance to the rapid glucoseinduced inactivation of maltose transport activity but not internalization and proteolysis. Glucose-Induced Ubiquitination of the ∆PEST Mutant Maltose Permease. Previous studies demonstrated that glucose stimulates ubiquitination of maltose permease and that this ubiquitination is involved in the glucose-induced degradation of maltose permease (5). If the PEST sequence is required for ubiquitin conjugation, we should expect significantly reduced rates of ubiquitination of the mutant protein. Ubiquitination of maltose permease is most effectively demonstrated in an endocytosis-deficient strain expressing abundant levels of a c-myc-tagged ubiquitin allele (5, 22). Strain CMY1052 contains a temperature-sensitive end3 allele and is deficient in endocytosis at the nonpermissive temperature of 37 °C (3, 23). Strain CMY1052 was transformed with plasmid pMyc-Ub carrying the c-myctagged ubiquitin gene expressed from the CUP1 copperinducible promoter (24) and either MAL61/HA or mal61/ HA(∆49-78). Transformants were grown under conditions optimized for demonstrating ubiquitinated Mal61/HA maltose Mutation of Maltose Permease FIGURE 3: Relative glucose-induced ubiquitination of wild-type and ∆PEST mutant Mal61/HA maltose permease. Strain CMY1052 was transformed with plasmid YEp105, pCUP1-mycUb [Medintz et al. (5)] carrying a c-myc-tagged ubiquitin allele expressed from the copper-inducible CUP1 promoter and with the indicated MAL61/ HA allele. Cells were grown in selective synthetic media with 2% maltose to early log phase and incubated in 0.1 mM CuSO4 for 3 h at 30 °C. Cultures were transferred to 37 °C for 1 h, and then 2% glucose was added to the growth media for an additional 30 min prior to harvesting. Samples were prepared for Western blotting as described in Materials and Methods and Figure 2. The arrow indicates the position of the monoubiquitinated Mal61/HAp. The positions of the molecular weight markers are indicated. permease (5). Samples containing equal amounts of maltose permease protein were analyzed by Western blot. The predicted molecular mass of Mal61/HA protein is approximately 69 kDa, but its apparent molecular mass is less, probably due to the hydrophobicity of the protein. As seen in Figure 3, a slower mobility species of Mal61/HAp is visible in the control lane, which our previous studies demonstrated to be a monoubiquitinated species of Mal61/ HA maltose permease (3). Only a single band is seen in the strain expressing Mal61/HA(∆49-78) permease with an apparent size consistent with the loss of 30 residues. Little or no ubiquitinated Mal61/HA(∆49-78) permease is evident at the appropriate molecular mass. The reduced rate of glucose-induced proteolysis of this mutant protein correlates with a reduced level of ubiquitination. InactiVation of Mal61/HA Maltose Permease Mutants in Pathway 1 and Pathway 2 Strains. Jiang et al. (17) identified two glucose sensing/signaling pathways in Saccharomyces that are used to monitor high-glucose levels and stimulate glucose-induced degradation of maltose permease. To determine whether N-terminal residues 49-78 are the target of glucose signaling pathways 1 or 2 or both, strains were constructed that carry a deletion of the chromosomal maltose permease gene and alterations in these signaling pathways. These were transformed with either plasmid-borne MAL61/ HA or mal61/HA(∆49-78) and underwent glucose inactivation assays. The panels to the left in Figure 4 show the results from strain CMY1050, genotype RGT2, in which both pathways 1 and 2 are functional. The results in the middle panels were obtained with strain CMY1051, genotype rgt2∆, which is isogenic to CMY1050 except that the RGT2 ORF is replaced with the KANR gene (15, 17). Since the gene encoding this high-glucose sensor of pathway 1 has been deleted, strain CMY1051 is deficient in pathway 1 signaling Biochemistry, Vol. 39, No. 15, 2000 4523 and only has a functional pathway 2. Introduction of the dominant constitutive RGT2-1 allele carried by plasmid pRGT2-1 into strain CMY1050 activates pathway 1 in the absence of glucose without activating pathway 2 (17). Results from strain CMY1050[pRGT2-1] are shown in the right column of panels in Figure 4. The results for each of these strains expressing wild-type Mal61/HA maltose permease are shown in the top row, and those for strains expressing Mal61/HA(∆49-78)p are shown in the bottom row. The top row of panels confirms the results of Jiang et al. (17), showing that pathway 1 is responsible for most of the observed rate of maltose permease proteolysis but not the rapid loss in transport activity. Despite the fact that maltose permease protein levels are decreasing in the RGT2-1 strain, maltose transport activity undergoes an approximately 2550% transient increase, also found by Jiang et al. (17). Pathway 2 is solely responsible for the rapid glucose-induced inactivation of maltose transport activity and has only modest effects on the proteolysis of maltose permease (17). In strain CMY1050 (RGT2) where both pathways 1 and 2 are active, little or no proteolysis of Mal61/HA(∆49-78) is stimulated by glucose. Also, in the constitutive pathway 1 strain (RGT21) no turnover of Mal61/HA(∆49-78) permease is detected. The effect of the ∆49-78 deletion is not as obvious in the rgt2∆ strain. Glucose induces a rapid loss in transport activity in wild-type permease, more rapid than can be explained by loss of permease protein, and this is dependent on pathway 2 signaling (3, 17). In the strain expressing only signaling pathway 2 (middle panels of Figure 4), one observes a significant decrease in this rapid inactivation in the strain expressing the ∆49-78 mutant permease. This suggests that the pathway 2 target sequence is also located in residues 49-78. DISCUSSION The predicted topology of Mal61p suggests that the N-terminal 109 residues, the C-terminal 65 residues, and the central region of 72 residues are each positioned on the cytoplasmic side of the plasma membrane and thus represent potential target sites for stimulating glucose-induced inactivation and proteolysis (2). The N-terminal cytoplasmic domain of the yeast uracil permease, Fur4p, contains a PESTlike sequence shown to be essential for turnover (11). Internalization of Fur4p was reduced when some of the serine residues in the PEST-like sequence were converted to alanines and severely impaired when all serines in this region were mutated or when this region was deleted (11). A PESTlike sequence, proposed to be a site of regulated phosphorylation and ubiquitination, is located in the N-terminal cytoplasmic domain of Mal61p (see Figure 1) (2). Other potential phosphorylation sites are found in each of the cytoplasmic domains, and lysine residues capable of serving as ubiquitination sites are positioned nearby these sites (14). A dileucine motif is found at residues 69 and 70 of Mal61p. A dileucine motif of GLUT4, the human insulin-responsive glucose transporter, was found to be required for internalization and also is proposed to play a similar role in the yeast Gap1 general amino acid permease and the eukaryotic β2adrenergic receptor (12, 25). Serine 295, located just after transmembrane domain 6, is essential for glucose-induced proteolysis of Mal61 maltose permease (14). Alteration of this residue to alanine had only a modest impact on the 4524 Biochemistry, Vol. 39, No. 15, 2000 Medintz et al. FIGURE 4: Glucose-induced inactivation of pathway 1 and pathway 2 deficient strains expressing mutant Mal61/HA maltose permease. Results of inactivation assays carried out in strain CMY1050 (RGT2), CMY1051 (rgt2∆), or CMY1050[pRGT2-1] expressing either MAL61/ HA or mal61/HA(∆49-78) are presented. Glucose was used to stimulate inactivation in the results shown in the left and middle panels (YNSG indicates inactivation media containing 2% glucose). The right column of panels presents inactivation assays carried out in 2% ethanol as the carbon source and represents effects of constitutive RGT2-1 signaling (YNSE indicates inactivation media containing 2% ethanol). Samples were taken at the indicated times, and the growth dilution (9), maltose transport activity (O), and relative Mal61/HA protein levels (b) were determined as described in Materials and Methods and Figure 2. glucose-stimulated loss of maltose transport activity (14), suggesting that S295 is not involved in the internalization of maltose permease but in a later event required for proteolysis. Glucose stimulates increased phosphorylation and ubiquitination of maltose permease, which are required for internalization and degradation (3, 5). We undertook a mutation analysis of sequences in the N- and C-terminal cytoplasmic domains of Mal61/HA to identify sites critical to the early steps of glucose-induced inactivation and to identify pathways by which glucose marks maltose permease for internalization and degradation. The cytoplasmic C-terminal regions of several yeast plasma membrane proteins contain sequences involved in their internalization, including Ste2 R-factor receptor, Gap1 general amino acid permease, and Ste3 a-factor receptor (6, 7, 13, 26). Alterations in the C-terminal cytoplasmic domain of Mal61 permease have variable but relatively minor effects on the rate of glucose-induced proteolysis (see Table 2). Mutation mal61/HA(NS581) produces a truncated protein with two proline residues, one serine and one threonine residue, and three lysine residues found in the last 15 C-terminal residues. This truncated protein is still sensitive to glucose-induced inactivation and proteolysis (Table 2). Mutations altering the proline residues at 566 or 577 to alanine, the potential protein kinase C phosphorylation sites at S572 and T573 to alanine, and all of the lysine residues at 569, 571, and 574 to alanine have little effect on the glucose-induced proteolysis of these truncated mutant permeases. These results suggest that residues in the C-terminal domain do not play an important role in glucose-induced proteolysis. The PEST sequence of Mal61 maltose permease is an important targeting sequence for the degradation of this protein. Mutant Mal61 proteins lacking this sequence, or portions thereof, exhibit significantly reduced rates of glucose-induced proteolysis (Table 3 and Figure 2). While we have not demonstrated the site(s) of ubiquitin conjugation, we do show a dramatic reduction in the relative amount of the ubiquitin-conjugated form of the Mal61(∆49-78) protein (Figure 3). Thus, residues 49-78 are required for efficient glucose-induced ubiquitination and proteolysis of maltose permease. Several lysine residues lie in and around the PEST sequence and could serve as ubiquitin conjugation sites. Since some effect is seen in deletion ∆2-30, it is likely that Mutation of Maltose Permease additional residues outside of the PEST(49-78) are also part of the target site. Two glucose signaling pathways regulate glucose-induced inactivation of maltose permease (17). Pathway 1 utilizes Rgt2p, a sensor of high concentrations of extracellular glucose that also regulates HXT1 expression encoding a lowaffinity glucose transporter (20). Pathway 1 stimulates better than half of the maltose permease proteolysis observed following glucose addition but does not contribute to the rapid inactivation of transport activity. RGT2-1 is a dominant allele causing constitutive expression of HXT1 (20, 27) and constitutive proteolysis of maltose permease, that is, degradation even in the absence of glucose (Figure 4) (17). Pathway 2 uses high rates of glucose transport and phosphorylation to generate an intracellular signal that is primarily responsible for the glucose-induced inactivation of maltose transport activity but also stimulates proteolysis (17, 28). Figure 4 demonstrates that proteolysis is entirely blocked in the ∆PEST mutant permease, clearly indicating that this region is the target of both pathway 1 and pathway 2 for stimulating glucose-induced proteolysis. Figures 2 and 4 show that N-terminal residues 49-78 are also required for the rapid glucose-induced loss of maltose transport activity. In strains expressing the ∆49-78 mutation or the dileucine motif mutation, the addition of glucose increases rather than decreases maltose transport activity. Kinetic analysis of the increased transport activity indicates that it results from an increase in Vmax with no change in Km. The simplest explanation is that the mutant permease proteins are able to continue through the secretory pathway and localize to the plasma membrane while the wild-type protein cannot. Glucose appears to block plasma membrane localization of wild-type permease or redirects it to a different subcellular site. This glucose-induced altered localization requires residues 49-78, or possibly just the dileucine motif. Consistent with this, Hu et al. (29) report a posttranscriptional block in maltose permease synthesis in the presence of glucose. These findings are reminiscent of the results that report differences in the sorting of Gap1p, the general amino acid permease, depending upon the nitrogen source (30). The transient increase in maltose transport activity observed following glucose addition to strains expressing the ∆49-78 mutation or the dileucine motif mutation is also seen in cells expressing wild-type permease but carrying RGT2-1 (Figure 4) (17) or glucose-treated cells containing a deletion of REG1 (31). In both of these strains, pathway 1 is active but pathway 2 is not. Thus, it appears that this increased transport activity is dependent on an activated pathway 1 but is observed only when pathway 2 is inactive or when the PEST region is deleted. Loss of the PEST region substantially reduces the glucose-induced rapid loss in transport activity in a strain expressing only pathway 2 (Figure 4). This suggests that residues 49-78 are the target of pathway 2 and, when acted upon or modified in the presence of glucose, allow maltose permease to be redirected away from the plasma membrane. Reg1p is an essential component of pathway 2 (31). Reg1p is a targeting subunit of protein phosphatase type 1 and binds to the catalytic subunit Glc7p in the presence of glucose (reviewed in refs 32-34). A reg1∆ strain exhibits reduced glucose-induced proteolysis of maltose permease and lacks the rapid glucose-stimulated loss of maltose transport activity Biochemistry, Vol. 39, No. 15, 2000 4525 (31). Previous studies demonstrated that Mal61/HA protein is phosphorylated in maltose-grown cells and that glucose stimulates increased levels of phosphorylated protein (3). On the basis of our observation that the relative level of maltose permease phosphorylation is reduced in reg1∆ strain (31), we suggest that Reg1p-Glc7p phosphatase indirectly stimulates the phosphorylation of PEST residues, via activation of a kinase intermediate. Evidence indicates that plasma membrane localized casein kinase I, encoded by YCK1 and YCK2, is responsible for the phosphorylation of the PESTlike sequence in uracil permease, stimulating ubiquitination and degradation of that permease (11, 35, 36). Casein kinase I is also reported to phosphorylate the SINDAKISS sequence of Ste2p (7). Preliminary experiments suggest a role for glucose-activated casein kinase I (37) in the glucose-induced inactivation of maltose permease (S. Liu and C. A. Michels, unpublished results). ACKNOWLEDGMENT We thank Sabira Ozcan and Mark Johnston for providing plasmids and disruption primers and Sara Danzi for technical assistance with in vitro mutagenesis. REFERENCES 1. Charron, M. J., Dubin, R. A., and Michels, C. A. (1986) Mol. Cell Biol. 6, 3891-3899. 2. Cheng, Q., and Michels, C. A. (1989) Genetics 123, 477484. 3. Medintz, I., Jiang, H., Han, E. K., Cui, W., and Michels, C. A. (1996) J. Bacteriol. 178, 2245-2254. 4. Hicke, L. (1997) FASEB J. 11, 1215-1226. 5. Medintz, I., Jiang, H., and Michels, C. A. (1998) J. Biol. Chem. 273, 34454-34462. 6. Hicke, L., and Riezman, H. (1996) Cell 84, 277-287. 7. Hicke, L., Zanolari, B., and Riezman, H. (1998) J. Cell Biol. 141, 349-358. 8. Berkower, C., Taglicht, D., and Michaelis, S. (1996) J. Biol. Chem. 271, 22983-22989. 9. Kolling, R., and Losko, S. (1997) EMBO J. 16, 2251-2261. 10. Rechsteiner, M., and Rogers, S. W. (1996) Trends Biochem. Sci. 21, 267-271. 11. Marchal, C., Haguenauer-Tsapis, R., and Urban-Grimal, D. (1998) Mol. Cell Biol. 18, 314-321. 12. Springael, J. Y., and Andre, B. (1998) Mol. Biol. Cell 9, 12531263. 13. Tan, P. K., Howard, J. P., and Payne, G. S. (1996) J. Cell Biol. 135, 1789-1800. 14. Brondijk, T. H., van der Rest, M. E., Pluim, D., de Vries, Y., Stingl, K., Poolman, B., and Konings, W. N. (1998) J. Biol. Chem. 273, 15352-15357. 15. Guldener, U., Heck, S., Fielder, T., Beinhauer, J., and Hegemann, J. H. (1996) Nucleic Acids Res. 24, 2519-2524. 16. Sikorski, R. S., and Hieter, P. (1989) Genetics 122, 19-27. 17. Jiang, H., Medintz, I., and Michels, C. A. (1997) Mol. Biol. Cell 8, 1293-1304. 18. Elledge, S. J., and Davis, R. W. (1988) Gene 70, 303-312. 19. Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. E., Seidman, J. G., Smith, J. A., and Struhl, K. (1998) Current Protocols in Molecular Biology, John Wiley and Sons Inc., New York. 20. Ozcan, S., Dover, J., Rosenwald, A. G., Wolfl, S., and Johnston, M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 1242812432. 21. Ozcan, S., Dover, J., and Johnston, M. (1998) EMBO J. 17, 2566-2573. 22. Hochstrasser, M., Ellison, M. J., Chau, V., and Varshavsky, A. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 4606-4610. 23. Raths, S., Rohrer, J., Crausaz, F., and Riezman, H. (1993) J. Cell Biol. 120, 55-65. 4526 Biochemistry, Vol. 39, No. 15, 2000 24. Ward, A. C., Castelli, L. A., Macreadie, I. G., and Azad, A. A. (1994) Yeast 10, 441-449. 25. Gabilondo, A. M., Hegler, J., Krasel, C., Boivin-Jahns, V., Hein, L., and Lohse, M. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 12285-12290. 26. Hein, C., and Andre, B. (1997) Mol. Microbiol. 24, 607616. 27. Marshall-Carlson, L., Neigeborn, L., Coons, D., Bisson, L., and Carlson, M. (1991) Genetics 128, 505-512. 28. Jiang, H., Medintz, I., Zhang, B., and Michels, C. A. (2000) J. Bacteriol. 182, 54-64. 29. Hu, Z., Yue, Y., Jiang, H., Zhang, B., Sherwood, P. W., and Michels, C. A. (2000) Genetics 154, 121-132. 30. Roberg, K. J., Rowley, N., and Kaiser, C. A. (1997) J. Cell Biol. 137, 1469-1482. Medintz et al. 31. Jiang, H., Tatchell, K., Liu, S., and Michels, C. A. (2000) Mol. Gen. Genet. (in press). 32. Johnston, M. (1999) Trends Genet. 15, 29-33. 33. Tu, J., and Carlson, M. (1995) EMBO J. 14, 5939-5946. 34. Tu, J., Song, W., and Carlson, M. (1996) Mol. Cell Biol. 16, 4199-4206. 35. Robinson, L. C., Menold, M. M., Garrett, S., and Culbertson, M. R. (1993) Mol. Cell Biol. 13, 2870-2881. 36. Vancura, A., Sessler, A., Leichus, B., and Kuret, J. (1994) J. Biol. Chem. 269, 19271-19278. 37. Estrada, E., Agostinis, P., Vandenheeds, J. R., Goris, J., Merlevede, W., Francois, J., Goffeau, A., and Ghislain, M. (1996) J. Biol. Chem. 271, 32064-32072. BI992455A