* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biophysics
Survey
Document related concepts
Transcript
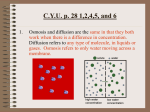
Introduction to biophysics Course Plan Course Contents Reference Books What is “Biophysics” Biophysics is a specialized sub area of biology It is the science of physical principles of life itself and of biological systems. Biophysics is an interdisciplinary science that explains the laws and principles of physics which govern various biological processes. Biophysics spans all levels of biological organization from molecular scale to whole organism Course Plan Quiz/Assignments: 10 marks Sessionals: 20+20=40 marks Terminal: 50 marks Course Contents Introduction to biophysics Basic concepts Osmosis, osmotic pressure, surface tension, diffusion, viscosity, thermal conduction, forces and energy Molecular structure of biological systems at a glance Energetics and Dynamics of Biological Systems Description of ATP, cell as an accumulator of electrochemical energy, energy consumption, respiration, mechanism of molecular energy transfer, thermodynamics and thermal molecular movement, mechanism of body temperature regulation, photosynthesis as a process of energy transfer and transformation, dynamics of blood flow, control of movement Course Contents Biological Membranes Membrane chemistry and structure, membrane physics, surface and interfacial tensions, diffusion and mobility of ions, electrostatic and mechanical properties of membranes Mechanical Properties of Biological Materials fluid flow, blood circulation, muscle contraction, swimming, flying etc Electric fields in cells and organisms Nerve Signals, nerve impulses, nervous system Physical factors of the environment Temperature, pressure, mechanical oscillations (vibrations, sound, hearing and hearing aids, effect of ultrasound), electromagnetic fields in the environment, ionizing radiations ebooks Textbook: Biophysics by P. S. Mishra, 2010 Reference books: Molecular And Cellular Biophysics by Meyer B. Jackson, Cambridge University Press, 2006 Biophysics by Roland Glaser, Springer, 2001 An Introduction to Med. Biophysics by Parveen Parkash Biological activities happening in different organs of living body like kidney, liver, heart, lungs as well as those in intracellular and extracellular biological fluid are governed by fundamental laws of physics namely Diffusion Osmosis Viscosity Surface Tension Osmosis The spontaneous passage of solvent from a solution of lower concentration towards a solution of higher concentration when the two are separated by a semi permeable membrane is called osmosis Osmosis is a special case of diffusion. It involves the diffusion of water through the semi permeable membrane to equalise the concentration of solutions on its two sides Osmosis in fresh and sea water fish Fresh water trout Sea water Herring wilting These cells are short of water; the tissue is limp and the plant is wilting Turgid plant The cells have taken up water by osmosis; the cells are turgid and the tissue is firm 25 Growth in a shoot tip these cells will divide cell division continues vacuoles forming cells absorb water by osmosis and expand Effect of different solutions on blood cells Osmosis Osmosis releases energy, and can be made to do work, as when a growing tree root splits a stone. Diffusion and Osmosis are both types of PASSIVE TRANSPORT - that is, no energy is required for the molecules to move into or out of the cell. Osmosis takes place due to difference in chemical potentials of water on two sides of membranes which leads to pressure gradient Solute decreases chemical potential of water. Water tends to flow from where its chemical potential is higher to where it is lower Reduced chemical potential causes reduced vapor pressure, lower freezing point and higher boiling point of the solution as compared with pure water Osmotic Pressure Osmosis may be opposed by increasing the pressure in the region of high solute concentration (hypertonic solution) with respect to that in the low solute concentration region (hypotonic solution). The hydrostatic pressure which just stops osmosis is the osmotic pressure The force per unit area, or pressure, required to prevent the passage of water through a selectively-permeable membrane and into a solution of greater concentration is equivalent to the osmotic pressure of the solution, or turgor. Osmotic pressure is a colligative property, meaning that the property depends on the concentration of the solute but not on its identity.