* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download H. Antifungal agents
Toxicodynamics wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
MTOR inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Drug discovery wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
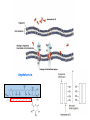
Antifungal agents Fungi Also known as mycoses Very large and diverse group of microorganisms Of major two types: yeasts and molds. Mycotic Infections: Cutaneous Subcutaneous Superficial Systemic: Can be life-threatening Usually occur in immunocompromised host Targets for Antifungal agents - Generally these targets should be different from mammalians. - Both human and fungi are eukaryotic, so not much difference could be found. - The most important difference is the presence of cell wall for fungi that is not found in humans. - Other targets are: - Inhibitors of DNA synthesis. Disruption of mitotic spindle. Interfere with metabolism. - The most exploited difference is the nature of sterols: - Important components of cell membrane for proper function of cell membrane enzymes and ion transporter proteins. Targets for Antifungal agents - Mammalians contain cholesterol while fungi posses ergosterol. - The difference is in the side chain of ergosterol which is more flat compared to cholesterol; - A difference that is responsible for providing selectivity for the majority of antifungal agents. Cholesterol Ergosterol Polyene membrane disrupters - Polyenes: macrocyclic lactones with distinct hydrophilic and lipophilic regions. - Produced from Streptomyces species - Hydrophilic: alcohol, carboxylic acids, sugar. - Lipophilic: contain a pharmacophore of 4-7 conjugated double bonds. - The number of the double bonds correlate directly to activity and inversely to toxicity. - Amphotericin is 10X more active which can be taken systemically. - Mechanism of action: have affinity to ergosterols containing membranes, then inserted in the cell membrane, disrupts its function, leak of cell components. Amphotericin Polyene membrane disrupters 1- Nystatin: - A tetraene agent that used topically. - No oral absorption, so used orally for mouth and GIT infections. 2- Amphotericin B: - Heptaene derivative with low enough toxicity for I.V use, but still toxic drug used with caution. - It does not cross BBB, so used intrathecally of brain fungal infections. - The main side effect is nephrotoxicity, reduced by formulation change. - Liposomal encapsulation was found to target infected tissues as the capillary size at the infected areas is larger, so release the drug specifically at that area. Nystatin Amphotericin nephrotoxicity Ergosterol Biosynthesis inhibitors A- Azoles: - The largest group of antifungal agents. - Some used topically and others used systemically. - some are orally bioavailable with broad spectrum properties. - SAR: - 5-membered ring with 2-3 Ns. - side chain attached to N. - At least has one aromatic ring. - Mechanism of action: - Act by inhibiting ergosterol synthesis by inhibiting CYP450 14α-demethylase, where the basic nitrogen N3 of the drug bind to heme iron of the enzyme blocking the active site. R R CO2 R Fe-Cyt P-450 CO2H Carboxylate Lanosterol N N Fe-Cyt P-450 Ergosterol N N Mechanism of action of Azoles Fe-Cyt P-450 Ergosterol Biosynthesis inhibitors - Inhibition will lead to accumulation of sterols with extra methyl group. - This new sterol structure does not have the shape and physical properties of the normal ones, leading to permeability changes. - Selectivity to mammalian C450 compared to the fungal one is 1:1000, and even, most of azoles considered mammalian C450 inhibitors which lead to serious drug-drug interactions in some cases. - Systemic azoles are : Ketoconazole, Itraconazole, Fluconazole and voricanazole. - Non-systemic azoles are : Clotrimazole, Oxiconazole and miconazole. Ergosterol Biosynthesis inhibitors 1. Ketoconazole: - Imidazole derivative, that is orally active for systemic infections. - Depends mainly on low stomach pH for absorption. - Inhibit C450 causing serious drug-drug interactions. - All its metabolites are inactive and mainly used topically. Ergosterol Biosynthesis inhibitors Ketoconazole Metabolism: - Deacylation. - Aromatic hydroxylation. Hydroxylation Binds heme active site Deacylation Ergosterol Biosynthesis inhibitors 2- Itraconazole: - Triazole derivative. - Oral bioavailability depend on food and stomach pH. - highly interfere with liver enzymes (serious drug drug interactions). 3- Fluconazole: - Equal bioavailability, oral and I.V. - Could cross BBB (Why?). - Weak inhibitor to some liver enzymes. Ergosterol Biosynthesis inhibitors B- Allylamines: - They have limited spectrum of activity which is only used for dermatophytes. - Includes: Naftifine, Terbinafine, and Tolnaftate - Mechanism of action: inhibit squalene epoxidase, - leading to build up of squalene that it self is toxic compound. - and also lead to reduction of sterol levels in cell membrane, leading to cell lysis. - Mammalian squalene epoxidase is less sensitive to these drugs. Squalene Epoxidase O Steps Steps HO HO Ergosterol Lanosterol Ergosterol Biosynthesis inhibitors -Terbinafine: - Topical and Oral. - Active against many dermatophytes. - Highly lipophilic. - Extensively metabolized. Ergosterol Biosynthesis inhibitors C- Morpholines: - Amorolfine: - The only drug of this class, used topically. - Act on Δ14 reductase enzyme, and Δ8, Δ7 isomerase enzymes. - produce non-similar compounds with different physical properties, leading to cell leakage. Miscellaneous mechanism of actions - Flucytosine: - Powerful agent for systemic infections. - Taken up by fungal cells and interferes with DNA synthesis - Prodrug to produce 5-flurouracil. - Griseofulvin: - Orally taken for superficial infections. - Not used topically. - Bind to protein tubulin. - Interfere with cell division. O O NH2 F N O N H Cytosine Deaminase HN O F HN F N H 5-Flurouracil O O P O O O N O OH 5-Flurodeoxyuridine monophosphate