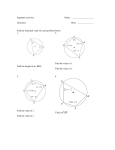
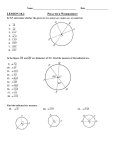
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Expression analysis of Arc in mouse brain Theresa Köhler
Epigenetics of human development wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Designer baby wikipedia , lookup
Microevolution wikipedia , lookup
Primary transcript wikipedia , lookup
History of genetic engineering wikipedia , lookup
Genomic imprinting wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Gene expression profiling wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Expression analysis of Arc in mouse brain Theresa Köhler Degree project in biology, 2006 Examensarbete i biologi 20p, 2006 Biology Education Centre and Institutionen för Fysiologi och Utvecklingsbiologi Supervisors: Reinald Fundele, Yang Yu Table of contents page Abbreviations………………………………………………………………………………. 3 Summary…………………………………………………………………………………… 4 Introduction………………………………………………………………………………… 5 • Parental behavior…………………………………………………………………... 5 • Parental behavior in rodents……………………………………………………….. 6 • Mechanisms of maternal behavior…………………………………………………. 6 • Olfaction…………………………………………………………………………… 6 • Brain regions important for maternal behavior………………………….………….7 • Hormonal control…………………………………………………………………... 8 • Sex hormones………………………………………………………………………. 8 • Other important factors…………………………………………………………….. 9 • Infanticide………………………………………………………………………….. 10 • Previous experiments………………………………………………………………. 11 • Arc…………………………………………………………………………………..12 • Quantitative Real Time PCR.……………………………………………….……... 13 • In situ hybridization.……………………………………………………………….. 13 • Immunohistochemistry…………………………………………………………….. 14 • Aims of the study………………………………………….……………..……….... 14 Results……………………………………………………………………………………… 15 • Papanicolaou tests………………………………………………………………….. 15 • Behavior tests………………………………………………………………………. 15 • Quantification of Arc in whole mouse brain………………………………………. 15 • Arc gene expression in mouse brain………………………………….……. ……... 16 • ARC protein localization in mouse brain…………………………………………...17 Discussion……………………………………………………………………..…………… 20 • Maternal behavior induces Arc…………………………………………………….. 20 • In situ hybridization experiments failed…………………………………………… 20 • Unclear role for ARC in maternal care…………………………………………... 20 • Reflection…………………………………………………………………………. 21 • Possible future work…………………………………………………………...…... 21 Materials and methods……………………………………………………………………... 22 • Laboratory animals……………………………………………………………….... 22 • Bacteria………………………………………………………………..…………… 22 • Primers…………………………………………………………………………….. 22 • Papanicolaou tests………………………………………………………………….. 22 • Behavior tests with exposure to pups……………………………………………….23 • Treatment and fixation of tissues…………………………………………………...23 • Quantitative reverse transcription PCR………………………………………….… 23 • Perfusion…………………………………………………………………………… 24 • Sectioning………………………………………………………………………….. 24 • In situ hybridization…………………………………………….………………….. 24 • Immunohistochemistry………………………………………….…………………. 27 Acknowledgements………………………………………………………………………… 28 References…………………………………………………………………………………..29 2 Abbreviations AP Arc BCIP CNS DAPI DEPC DIG DTT EA 50 EDTA Egr1 FITC HCl IEG IPTG LB-medium LiCl MgCl2 MPOA M-MLV NaCl NBT NT NTMT O.G.6 PBS PFA qRT-PCR SSC TBS VTA X-gal Alkaline phosphatase Activity regulated cytoskeletal-associated protein 5-bromo-4-chloro-3-indolyl phosphate Central nervous system 4',6-Diamidino-2-phenylindol Diethylpyrocarbonate Digoxigenin Dithiothreitol Eosin-Azure 50 Ethylenediaminetetraacetic acid Early growth response 1 Fluorescein isothiocyanate Hydrochloric acid Immediate early gene Isopropyl β-D-1-thiogalactopyranoside Luria Bertani-medium Lithium chloride Magnesium Chloride Medial preoptic area Moloney murine leukemia virus Sodium Chloride Nitro blue tetrazolium chloride NaCl-Tris buffer NaCl-Tris-MgCl2-Tween-buffer Orange G-6 Phosphate buffered saline Paraformaldehyde Quantitative Reverse Transcription PCR Standard saline citrate Tris buffered saline Ventral tegmental area 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside 3 Summary Arc, an immediate early gene, expressed in the brain of mice encodes the activity regulated cytoskeletal-associated protein (ARC) that is found on dendrites of neurons. It regulates cell development and plays an important role in neuronal plasticity and formation of long-term memory. Arc is induced in various situations of neuronal activity. In previous experiments it was found differentially expressed in female virgin mice exposed to pups compared to mice without exposure. This lead to the conclusion that maternal behavior might be an inductor for Arc expression. To support this hypothesis I carried out behavior tests with mice and their brains were dissected for analytical research about Arc expression and ARC protein localization. Quantitative reverse transcription polymerase chain reaction, in situ hybridization and immunohistochemical staining were performed. These showed slow but constant increase of Arc mRNA after pup exposure, so it can be assumed that one feature of maternal behavior is induction of Arc. The ARC protein was mainly found in the cortex, medial preoptic area (MPOA) and brainstem with no significant differences in brains without exposure to pups compared to brains with such exposure, suggesting an unclear role for ARC in maternal care. 4 Introduction Parental behavior Instinctive care for offspring is widely spread in nature. One type of brood care can be found in animals like insects and spiders. Ants, termites, wasps and bees protect their larvae by building nests and offer nutrition. Digger wasps, for example, paralyze insects and carry those to the nest to feed larvae (Bellmann, 1995). Carrion beetles produce balls of dead meat to put their eggs on (Freude et al., 1971). Scorpions, belonging to the class of Arachnida, are special, because they, in contrast to other spiders, are viviparous and carry their offspring on their back (Polis, 1990). Vertebrates have evolved improved methods of parental behavior. Roughly, the fewer the number of eggs or offspring the more intensive the care. Females of the common frog, Rana temporaria, lay up to 4000 eggs into the water and let them just float (Kuzmin et al., 2004). In contrast, Dendrobates pumilio, a type of poison dart frog, lays just three to five eggs and, after hatching, the tadpoles are transported to water retaining locations and fed with unfertilized eggs (Donnelly, 1989). Males of midwife toads carry strings of fertilized eggs around their legs (Vogt, 1842). Females of the extinct frog Rheobatrachus, used to breed their eggs inside the stomach inhibiting gastric secretion (Pough et al., 2003). Reptiles exhibit different parental behavior. Female crocodiles build a nest that is guarded during incubation, and after hatching pups are carried to the water and protected. Most turtles put their eggs in sandpits, let the sun incubate and leave the nests. Birds have developed a wide spectrum of nest building. As they are often monogamic usually both parents are responsible for incubation of the eggs. However, some ratites let the sun incubate their eggs and male nandus breed the eggs and care for the hatchlings alone (Pough et al., 2005). Of all vertebrates, mammals have developed the most elaborate methods of maternal care. As they are viviparous, a very special characteristic of mammals is internal incubation of offspring in gestation. The formation of a chorioallantoic placenta contributes to the fitness of the young, as this is the organ that supplies nutrition. It is also essential for gas exchange and removal of waste products of the developing fetus (Rossant & Cross, 2001). Another important feature of mammals is lactation, which is provoked by a change in hormone levels and necessary for provision of nutrition to the young after birth. Postnatal care like pup retrieval, nursing, feeding, crouching, licking, nest defense and nest building with provided material is a feature of a special mother-infant relationship. Especially retrieving of the young, that is carrying the pups with the mouth back into the nest, is a widely observed behavior pattern among mammals. Amongst others it can be found in dogs, cats, guinea pigs, golden hamsters and rats (Shi et al., 2005). There are two important groups of mammals displaying maternal behavior. The altricial ones, including mice, rats, hamsters, rabbits, cats and dogs, are born rather undeveloped, often blind and deaf with insufficient body control and dependent on aid and protection by their parents. Because of their immaturity they are bound to the nest seeking for warmth and food. To stimulate excretion of urine and feces the mother has to lick the anal-genital region of the offspring. In case of danger, the mother defends the young against intruders (Shi et al., 2005). On the other hand there are precocial mammals, mainly ungulates living in large, grazing and moving herds, but also whales. They are put into world relatively mature and leave the nest right after birth to start exploring the environment with their already developed senses. However, the mother still accompanies the young and provides nutrition and protection (Shi et al., 2005). 5 Parental behavior in rodents Rodents, especially rats and mice as they are easy to handle, are good model objects to study behavior and genetics of mammals. Females carry out the above mentioned parental care after pregnancy. Eventually also males exhibit crouching, licking and retrieving behavior. In captivity also virgin females that have never been in contact with pups before are likely to care for the young of other mice. It has even been reported that rodents adopt pups foreign to their species, e.g. rats and golden hamsters raising offspring of mice together with their own young post parturition (Lonstein & De Vries, 2000). So it can be concluded that the stimuli activating the parental behavior are found in different species. However, although many experiments have been performed, the stimuli causing the retrieval behavior have not been detected yet. Presumably smell is involved, because even dead pups can be retrieved (Gandelmann et al., 1970), so sounds and movements can be excluded as primary stimuli. Though there are some exceptions, brood care in rodents is mainly carried out by females (Lonstein & De Vries, 2000). Thus, I will focus on maternal behavior in this report. Mechanisms of maternal behavior A wide range of factors and complex brain interactions influences maternal behavior. The endocrine system prepares the uterus for implantation and controls steps of gestation, but it also plays an important role in initiation and maintaining of nurturing behavior as well as in regulation of gene expression and processing environmental stimuli (Kendrick et al., 1997). Vaginocervical stimulation promotes maternal behavior in sheep (Poindron et al., 1987) and in rats (Kendrick et al., 1997; Insel and Young, 2001). β-endorphin, an opioid synthesized in the hypothalamus and pituary gland, regulates pain, hunger and production of sexual hormones (Simantov and Snyder, 1976). This β-endorphin increases in the placenta during conception and labor in humans while it can be found throughout pregnancy in the limbic brain (hypothalamus, amygdala and midbrain) of rats (Keverne, 1988). The role of opioids is still not clear as experiments with opioid receptors in the medial preoptic area (MPOA) revealed a suppressive function of µ-opioids and morphins for parental behavior (Gulledge et al., 1999). However, one should always consider the way females are brought up, as experiences in their life significantly can affect their behavior. Thus it was found that rats separated from their mother as juveniles exhibited deficits in maternal behavior in adulthood, such as decreased crouching and licking (Lovic et al., 2001). Epigenetic traits may also be involved in brood care, especially in passing on maternal behavior over generations. Champagne proposed a relationship was between maternal care, cytosine methylation and gene expression. Offspring that had experienced low levels of crouching and licking was compared to offspring that had experienced higher levels. The former were found to exhibit increased methylation of estrogen receptor-α1b promotor, which finally lead to differential estrogen receptor expression in MPOA (Champagne et al. 2006). Olfaction All throughout the fauna the olfactory sense is involved in regulating sexual behavior and reproduction. Even in invertebrates the use of sex pheromones has been described. Tests with deaf female mice support the hypothesis of olfaction being the main sense to stimulate maternal behavior. Comparing deaf and normal-hearing mothers no differences in maternal care were reported (D’Amato and Populin, 1986). Neurotransmitters play important roles in mediating environmental signals to the brain. Neurotransmitters are small molecules that transfer electrical signals from one neuron to the 6 other. They can be categorized as amino acids, peptides and monoamines (Lehninger et al., 2001). Noradrenalin is synthesized in the adrenal gland and in the locus coeruleus in the central nervous system (CNS) and functions as hormone as well as neurotransmitter in the sympathetic nervous system (Lehninger et al., 2001). Experiments have shown that noradrenalin is necessary for pup recognition and depletion of noradrenalin in the olfactory bulb leads to cannibalism and disturbance of maternal behavior (Calamandrei et al., 1992). Gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter of the CNS, and glutamate are mainly involved in olfactory learning and establishment of long-term memory. Glutamic acid is an amino acids but also acts as an inducing neurotransmitter in the CNS. Changes in release of those molecules have been observed during parturition in olfactory bulb (Levy et al., 2004). Tests have shown that females in most mammalian species find the smell of progeny aversive. Non-pregnant, non-lactating females usually avoid placenta and amniotic fluids covering the pups. So it can be concluded that the brain, especially the olfactory bulb, has to undergo drastic changes to accept offspring. The once repelling odors turn into signals for recognition of the young. One major molecule found to attract females is dodecyl propionate (Levy et al., 2004). Licking the anogenital region of pups is widely spread among rodents (Shi et al., 2005). This can be seen as a mechanism to take up the smell of the offspring, but it is also a way to distinguish the sex of the young and it stimulates urination and defecation of pups. Another reason might be for the female to take up fluids and salts to maintain her water homeostasis. Brain regions important for maternal behavior Behavior of any kind involves multi-sensory processing and complex interactions between a large number of organs, cells and molecules. The brain is essential to control transfer of information. After decades of brain research, special areas have been found to be indispensable for maternal behavior. The medial MPOA is located in the forebrain rostral to the hypothalamus (Figure 1). M idbr ai n H ip p o c a mpu s C er e be llu m Co r t ex O lf a c t o r y bu lb B ra in st em / Medu ll a S t r ia t u m Pons T ha la m u s B a s a l Fo r e bra in w it h MPO A S e ptu m A myg da la a nd h y p ot ha la mu s Figure 1. Regions of the mouse brain from geneatlas.org with permission from the copyright-holder 7 As discovered in rats, the MPOA is rich in endogenous opioid receptors (Gulledge et al., 2000). The pathways that these receptors innervate are pivotal to the expression of maternal behavior by stimulating hormones like estrogen. The MPOA and the androgen sensitive neurons that form the ventral amygdalofugal pathway leading to the medial preoptic nucleus pass on information concerning sexually relevant stimuli to the brain. Lesions to this brain region severely impair the full expression of sexual and parenting behaviors. Damage in MPOA evoke disruption of placentophagia, delay start of crouching and pup licking and inhibit pup retrieval and nest building (Franz et al., 1986; Numan, 1987). On the other hand, injections of estradiol, a steroid hormone inducing development of female sex organs, in the MPOA promote maternal behavior (Numan, 1987). Another important structure is the olfactory bulb in the forebrain (Figure 1) of vertebrates. It is mainly involved in olfaction. The olfactory bulb receives input directly from the olfactory nerve and its output reaches the amygdala, a part of the limbic system very active in processing emotions and memories, and the hypothalamus (Figure 1) where it influences and defines animal behavior. Surprisingly, lesion studies in rats and sheep have revealed that damage in the olfactory bulb ease onset of maternal behavior, probably by helping females defeat their inborn objection to progeny smells (Insel and Young, 2001). The bed nucleus of the stria terminalis, a bundle of nerves connecting the amygdala to the hypothalamus that is implied in anxiety and stress response, also seems to play a role in maternal behavior (Mark et al., 1995). Lesion studies in the ventral tegmental area (VTA) in rats propose a facilitating influence of this region to mother-infant behavior. The VTA is situated in the midbrain (Figure 1) and rich in dopamine and serotonin receptors. It is considered to comprise a variety of functions in emotions and behavioral motivation (Insel and Young, 2001). After injuries of cingulated cortex, the cortical part of the cingulated gyrus, and the amygdala, impaired behavior between mother and infant was noted (Kendrick et al., 1997). In the forebrain subventricular zone new synthesis of progenitor neuronal cells induced by mating was described in mice. Those progenitors migrate to produce new olfactory cells possibly to prepare the female for olfactory recognition of pups. This process is mediated by the hormone prolactin (Shingo et al., 2003). Hormonal control Hormones are biochemical messengers that mediate the transfer of signals between different types of cells and organs in the body. In contrast to neurotransmitters, hormones are distributed by the blood and thus their effects may occur later and last longer. They are synthesized by endocrine glands and can be recognized only by cells with specific receptors. After docking to such receptors, second messengers are released inside the cell to turn on cascades of reactions and to transfer the signal to the final destination. Only steroid hormones are able to penetrate the cell membrane to form hormone–protein-complexes in the cytoplasm and finally act directly on the DNA inside the nucleus. The hormonal system regulates many functions and reactions of the body, e.g. the reproduction system, growth and development, the immune system and metabolism (Lehninger et al., 2001). Presumably the initiation of maternal behavior is assumedly controlled by hormonal changes, especially during the last days of gestation. Sex hormones During pregnancy mice undergo a large number of hormonal alterations. The level of progesterone drops at the last day of gestation, while estrogen and prolactin levels rise just before parturition. In contrast to the human menstrual cycle, which takes 28 days, the estrus 8 cycle of the mouse is just 5 days. If conception does not occur, the endometrium will be reabsorbed instead of being shedded as in menstruation. The estrus cycle starts with the proestrus, when follicles start to grow in the ovary and the endometrium develops under the influence of estrogen. The estrus, also called “heat”, is the phase of sexual reception, the genital tract red and swollen. Still under regulation of estrogen and gonadotropic hormones, follicles mature and ovulation occurs. In the following phase, the metestrus, estrogen levels decline and the corpus luteum forms while progesterone levels increase. In case of pregnancy, diestrus ensues the production of progesterone, otherwise the corpus luteum will degenerate (Shi et al., 2005). These neuroendocrine factors are believed to be associated with nurturing behavior. Experiments have shown that female rats tend to attack pups during gestation while directly after birth displaying perfect maternal performance. Additionally the neuropeptides prolactin and oxytocin, both participating in mammal lactation, are implicated in influencing parental response to progeny (Rosenblatt et al., 1987; Kendrick et al., 1997). Prolactin can induce brood care in some species, as demonstrated in rodents (Rosenblatt et al., 1987; Kendrick et al., 1997). Mice with mutations in the prolactin receptor gene show disrupted maternal retrieval and nest building (Ormandy et al., 1997; Lucas et al., 1998). Oxytocin is produced in the hypothalamus and transferred and stored in the pituitary gland. It is known to affect sexuality and the relationship between mother and infant (Lehninger et al., 2001). It seems to work in favor of maternal behavior (Kendrick et al., 1997), in fact, to inhibit avoidance of pups. Addition of oxytocin to olfactory bulb, MPOA and VTA lowers processing of odors and promotes nurturing care as tested in sheep and rats (Insel and Young, 2001). Those findings suggest that acceptance of pups can be accomplished by decrease of olfactory abilities, which is mediated by up-regulation of oxytocin (Insel and Young, 2001). Estrogen, a steroid hormone regulating the estrus cycle and strongly influencing the female’s sexuality, has been found to be involved also in promoting maternal behavior, e.g. in sheep (Poindron et al., 1987). Mice with mutations in the estrogen receptor gene show decreased parental behavior towards young and exhibit infanticide more frequently than wildtype mice (Ogawa et al., 1998). Other important factors Knockout studies have revealed a significant number of genes believed to play important roles in maternal behavior. Female mice lacking forkhead box B1 (Fkh5), specifically expressed in the developing CNS, fail to nuture offspring (Wehr et al., 1997). Mice with deletions in the testicular orphan nuclear receptor 4 (Tr4), with not yet known function, show incomplete reproduction and maternal behavior (Collins et al., 2004). By disrupting the dopamine beta hydroxylase (Dbh) gene, mice lacking norepinephrine and epinephrine were created. Those do not perform pup retrieval (Thomas and Palmiter, 1997). Studies with mice carrying a germ line null mutation of the prolactin receptor, interacting in regulation of maternal behavior, have revealed impaired pup-induced maternal behavior (Ormandy et al., 1997; Lucas et al., 1998). The methyl-CpG binding domain 2 (Mbd2) gene codes for a transcriptional repressor that binds to methylated DNA. Null mutants for this gene exhibit defective maternal behavior (Hendrich et al., 2001). Paternally-expressed gene 3 (Peg3) is an imprinted gene expressed in different embryonic tissues and adult brain. Mutations in this gene lead to growth retardation as well as impaired maternal care (Li et al., 1999). For Paternally expressed gene 1 (Peg1), also known as Mest, a role in adult behavior is suggested as disrupting this gene by gene targeting results in abnormal maternal behavior and impaired placentophagia (Lefebvre et al, 9 1998). These genes, Mbd2, Peg1 and Peg3, demonstrate the importance of epigenetic influence on maternal behavior. Heterotrimeric G proteins of the Gq/11 family are suggested to mediate neuronal activation in MPOA, the bed nucleus of stria terminalis and the lateral septum after pup exposure. Disruption of the Gq/11 gene results in mice having normal olfaction and motor behavior, but exhibiting impaired maternal performance such as lack of nest building and crouching (Wettschureck et al., 2004). It is known that immediate early genes (IEGs) are activated in response to contact with pups. Sensory stimuli, such as visual triggers, odors and sounds, can turn on the expression of IEGs directly after exposure and may last for several hours (Brown et al., 1996). Constitutive transcription of IEGs usually is low (Sheng and Greenberg, 1990). In ewes the IEGs C-fos and Zif/268, also known as early growth response 1 (Egr1), are stronger in the olfactory bulb and parts of the cortex after exposure to lambs (Levy et al., 2004; Da Costa et al., 1996). Genes belonging to the FBJ osteosarcoma oncogenes (Fos) family have shown to be expressed in many brain regions, such as cortex, hypothalamus, thalamus, limbic system and brainstem (Brown et al., 1996) (Figure 1). Those areas are responsible for a wide variety of behavior patterns and also for maternal behavior (Kendrick et al., 1997). Knockout of the FosB gene in mice results in deficient nursing behavior though sensory and cognitive abilities are not affected (Brown et al., 1996). Infanticide Infanticide means intentional killing of progeny of the same species. It is widely spread in mammals such as carnivores, primates, dolphins and rodents but can also be found in birds. Reasons for this behavior can be diverse (Hausfater and Hrdy, 1984). Sociobiological observations have revealed that male lions kill foreign offspring to preserve and pass on their own alleles. Female lions also tend to kill alien pups, but in contrast to the males their goal is to reduce the population pressure to secure survival of their own progeny (Wehner and Gehring, 1995). Male bottlenose dolphins have been reported to kill other dolphins that are less than one year old. Scientists, still at a loss with this phenomenon, assume that killing the calf might bring the female dolphin into a receptive state sooner (Milius, 1998). Frequent disturbance and threat by predators can cause infanticidal behavior as well. During the time of bombings in Yugoslavia by the NATO in 1999 different animals in the Belgrade Zoo, among those a tigress and a female wolf, have been reported to be so traumatized, they ate their own offspring (Rodgers, 1999). Factory farming also can lead to cannibalism as domestic sows have been seen to eat their newborn (Fox and Coats, 1989). In mice and rats overpopulation can provoke infanticidal behavior. Stressed adult rodents kill their young and often eat them. Especially feral virgins and elderly female mice are known to exhibit infanticide when exposed to pups. This is done by biting, bruising, attacking, and in the end killing the young. In contrast, laboratory strains usually do not show this kind of aggression because of selection against aggressiveness by laboratory breeding conditions. In principle, infanticide is increased in late pregnancy as shown in wild-type female house mice. After giving birth the mothers show perfectly maternal behavior (Shi et al. 2005). Injections of the peptide hormone oxytocin under the skin can prevent infanticidal behavior in the last days of gestation, implying that hormones are involved in this. Similar treatment with prostaglandin F2α, a hormone consisting of unsaturated fatty acids and known to regulate birth and parturition, stops infanticide in previously infanticidal pregnant mice but does not show any effect in previously infanticidal virgin females (McCarthy et al., 1985). 10 In some stocks of mice exogenous applied testosterone, an androgen steroid hormone with main functions in sexuality, can induce infanticide (Gandelman & Vom Saal, 1977). Previous experiments In previous work in this laboratory (Shi et al. 2005) F1 hybrid virgins (Mus musculus x Mus spretus) underwent behavior tests. These F1 hybrids were 99% genetically identical because the parental strains were inbred. Therefore differences in their behavior patterns are results of epigenetic and developmental effects. 31 virgin F1 mice were tested for maternal behavior, which includes pup retrieval and nest building when exposed to foreign offspring. Among those virgin females, nine exhibited infanticidal behavior, attacking and killing the pups. The global gene expression was examined by microarray hybridization (Shi et al. 2005). Microarrays, also called gene chips, are microscope slides on which short oligonucleotides (25 - 70 bp), each representing a gene, are applied. Tagged probes can bind to the immobilized sequences on the arrays and emit signals. Nowadays microarrays are widely used in genome analysis, diagnostics and for investigations of differentially expressed genes (Müller and Röder, 2004). This method is intended to assess and compare amounts of mRNA in different samples. Gene chips containing 13000 mouse sequences were used and gene expression of four different test groups was compared. Brains of normal F1 females that had shown perfect maternal behavior with and without exposure to pups, and brains from infanticidal mice with and without exposure to pups were examined (Shi et al. 2005). Outcomes are shown in figure 2. Maternal after exposure 59 ↑ 71 ↓ Egr1, Dnmt3a Infanticidal after exposure Hbb-bh1 12 ↑ 2 ↓ 2 ↓ 37 ↑ Hbb-bh1, Arc, Egr1 Maternal w/o exposure 7 ↑ Cbx5 7 ↓ Infanticidal w/o exposure Figure 2. Differential gene expression in brains of F1 female mice exhibiting infanticidal vs. normal maternal behavior towards alien pups, with and without exposure to pups. Up-regulation (↑) and down-regulation (↓) of genes is with reference to basal levels indicated by direction of the arrow. Thus, “maternal, no exposure” represents the basal level of gene expression; 2 genes are down- and 37 up-regulated in “maternal, exposure” (Shi et al., 2005). 11 From those experiments it could be concluded, that Egr-1, Cbx-5, Hbb-bh1, Dnmt3a and Arc are involved in maternal behavior (Shi et al., 2005). Egr1, early growth response 1, was downregulated in infanticidal F1 females and upregulated in good mothers after exposure to pups. It encodes a DNA binding protein that regulates transcription. Mice with mutations in this gene show memory defects, but the IEG Egr1 is also believed to play a role in situations of increased stress and to interact with Arc (Li et al., 2005). Dnmt3a, also downregulated in infanticidal females, codes for DNA methyl transferase 3A that is responsible for establishment of methylation patterns (Fatemi et al., 2002). This downregulation correlates with results indicating a strong role for epigenetics in maternal behavior. It was observed that pup licking and grooming induces methylation of glucocorticoid receptor (GR) gene promoter in the hippocampus of rats and binding of EGR1 to the GR promotor (Weaver et al., 2004). The protein of Cbx5, chromobox homolog 5, binds to chromatin and is therefore assumed to regulate differentiation and epigenetics. Hbb-bh1, hemoglobin Z, was upregulated in females showing maternal behavior. Enzymatic hydrolysis of different chains of blood protein hemoglobins generates hemorphins, opioid peptides, which may also play a role in behavior (Nyberg et al., 1997). Arc The activity regulated cytoskeletal-associated protein (ARC) belongs to the group of IEG. Those proteins activate cascades of downstream genes inducing delayed response genes whose gene products themselves switch on late response genes to control long-term cellular response. About 40 IEGs identified so far can be classified into regulatory genes that mostly act as transcription factors and into effector genes that encode proteins and act directly at the synapse(Lanahan and Worley, 1998). They regulate cell development and function as well as neuronal plasticity and formation of long-term memory (Sheng and Greenberg, 1990). Arc was first discovered in rat (Lyford et al., 1995). It has also been named Arg3.1 (Link et al., 1995) and homologous genes have been found in human and mouse (Lyford et al., 1995). Unlike other IEGs, e.g. C-fos, JunB and Egr1, that encode transcription factors, ARC is a cytoskeletal protein that is mainly localized in neuronal dendrites (Lyford et al., 1995). The ARC protein shows high similarity to the protein α-SPECTRIN (Link et al., 1995), also a cytoskeletal component of the cell, and has been described to bind to F-ACTIN (Lyford et al., 1995). F-ACTIN is a polymer responsible for structure and stabilization of the cell, but also one of the main actors in muscles. Arc mRNA is weakly but detectably expressed in adult organs like kidney, stomach, liver, spleen, lung, muscles and heart (Link et al., 1995). Higher basal levels can be found in the brain, where Arc is strongly expressed in the cortex (Link et al., 1995). Constitutive expression has been detected also in lower levels in the striatum, hippocampus, reticular thalamic nucleus and cerebellar cortex (Figure 1) (Ons et al., 2004) as well as in caudate putamen (Link et al., 1995). Immunohistochemistry assessed weak protein expression in the hippocampus, amygdala, hypothalamus, striatum and cortex (Figure 1) with highest amounts in neuronal layers II, III, IV and VI (Lyford et al., 1995; Link et al., 1995). Additionally, Arc seems to be necessary for regulation and patterns in embryogenesis. This has been shown in experiments with Arc knockout mutant mice. Growth was retarded and gastrulation was completely disrupted and followed by death of the embryo on day 8.5. Arc is expressed widely spread throughout all tissues in early mouse embryo (Liu et al., 1999). Arc plays a very important role in learning processes, longterm potentiation and brain plasticity that demand synthesis of new mRNA and proteins. Studies of psychomotor drugs have revealed that Arc transcription is induced in striatal neurons by cocaine (Fosnaugh et al., 12 1995) and amphetamine that both alter release of neurotransmitters (Tan et al., 2000). In adult rats, Arc mRNA was upregulated after exposure to a multifarious environment in the cortex, especially in layers III and V, the hippocampus and striatum (Figure 1) and to a lower extent in the dentate gyrus (Pinaud et al., 2001). Stress such as a new environment, forced swimming or immobilization was also found to initiate Arc expression in the brain (Ons et al., 2004). In agreement with these results, Arc expression is reduced in overtrained rats compared to rats trained for the first time (Kelly and Deadwyler, 2002). In mice, Arc is possibly involved in regulating the circadian rhythm as it appears in great quantities in the suprachiasmatic nucleus, reaching its climax 30 – 60 min after light exposure in a dark phase (Nishimura et al., 2003). Arc expression is mediated by activation of N-methyl-D-aspartate (NMDA) receptors that are usually activated by glutamate and involved in transmission of neuronal signals (Steward and Worley, 2001). If NMDA receptors are blocked, basal expression levels of Arc in brain are reduced (Link et al., 1995). Intrahippocampal administration of Arc antisense oligodeoxynucleotides inhibits expression of ARC and thus impairs learning (Guzowski et al., 2000). From these results it can be concluded, that ARC is involved in processing information and that it is increased in situations of high neural activity (Lyford et al., 1995; Link et al., 1995). Recently it has been discovered, that Egr transcription factors interact with Arc mRNA. Egr1- and Egr3-deficient mice were used to study the role of IEGs and brains lacking one of those genes show disturbed Arc expression, while brains lacking both reveal complete absence of Arc (Li et al., 2005). Quantitative Reverse Transcription PCR Quantitative Reverse Transcription PCR (qRT-PCR) is a method based on conventional PCR. It is used for mRNA expression studies and for quantification of cDNA. The “rotor gene” qRT-PCR machine (RG-3000, Corbett research, Sidney, Australia) measures the concentration of nucleic acids after each PCR cycle. Intercalating DNA dyes, e.g. SYBR Green or ethidium bromide, are used to detect signals in the sample. Fluorescent SYBR Green, with excitation and emission maxima at 494 nm and 512 nm, binds to double-stranded DNA molecules and thus allows measuring of DNA amplification after each cycle. The fluorescent signal increases proportionally to the amount of PCR product after each round of PCR (QuantiTect SYBR Green PCR Kit, Technical Manual; Higuchi et al. 1992). A melting curve analysis can be performed to assure that fluorescent signals trace back to the specific product. Possible primer dimers that would disturb the results can be excluded this way. The melting curve is established by increasing the temperature very slowly from low to high, so that double stranded DNA denatures and the fluorescence decreases. As the melting point of a specific DNA product depends on its length and base composition, the bulk of DNA in the sample will denature at a specific temperature, displayed by a peak in the curve. Diverse peaks or plateaus indicate nonspecific products, primer dimers and impurity (QuantiTect SYBR Green PCR Kit, Technical Manual; Higuchi et al. 1992). Comparative quantification is carried out with help of a reference gene that is expressed equally in all tissues and organs, e.g. the housekeeping gene encoding glyceraldehyd-3phosphate dehydrogenase (gap-dh) or tubulin. The ratio between the amounts of reference and target mRNA can be calculated, so differential gene expression can be compared in different samples (QuantiTect SYBR Green PCR Kit, Technical Manual; Higuchi et al. 1992). 13 In situ hybridization In situ hybridization is a method for analysis of gene expression in tissues or cells by detection of mRNA. The basis is the characteristic of DNA- or RNA-strands to bind to complementary strands by establishment of hydrogen bonds. So single-stranded probes, usually tagged to digoxigenin, which can be easily attached to oligonucleotides, can be applied to the tissue to be examined and hybridize to target-strands. An antibody against digoxigenin is used for visualization of gene expression. This antibody itself is tagged to a fluorescent molecule or a reporter enzyme, so signals of cells with active gene transcription can be observed and localized by microscope. Antisense and sense probes, corresponding to short parts (200 - 600 bp) of the Arc gene have been established. Antisense is complimentary to a part of the target mRNA and supposed to bind specifically to emit signals. Sense, used as control-probe, is complimentary to antisense and thus should not attach to RNA to be detected (Jin & Lloyd, 1997; Leitsch, 1994). For hybridization the tissue has to be fixed. This can be done with paraformaldehyde (PFA), a polymer of formaldehyde, which is used to cross-link proteins and DNA reversibly. If the antibody is coupled to the enzyme alkaline phosphatase (AP), endogenous AP, released by cells of the brain, has to be inactivated by acid treatment. Use of proteinase K catalyzes the hydrolysis of peptide bonds for degradation of proteins to improve accessability for probes to mRNA. Acetylation of slides with acetic anhydride buffer is performed to destroy RNAses that might decompose the RNA to be detected. For hybridization it is essential to keep the labeled DNA probes denatured. This is ensured by addition of formamide, a methanamide. Blocking agents are used in the hybridization mix to inhibit unspecific binding (Jin & Lloyd, 1997; Leitsch, 1994). After hybridization various washing steps have to be achieved to remove unspecific binding of the labeled probe. Anti-digoxigenin antibody, usually linked to AP, is applied, to bind the probe. AP transfers a phosphate from 5-bromo-4-chloro-3-indolyl phosphate (BCIP) onto nitro blue tetrazolium chloride (NBT) resulting in a purple precipitate. By this color reaction, brain regions that contain cells expressing the gene to be analyzed can be made visible, because AP shows its enzymatic activity just at sites of target mRNA (Jin & Lloyd, 1997; Leitsch, 1994) . Immunohistochemistry Immunohistochemical staining is a common biological technique for detection of proteins in tissues. The protein to be localized serves as antigen that can be recognized and bound by a specific antibody. To create a visual signal a secondary antibody directed against the primary antibody is applied. This secondary antibody is usually conjugated to a reporter enzyme like AP or to a fluorescent molecule. It can also be labeled with biotin, which allows another step of signal enhancement with avidin or streptavidin. Tissues are fixed and acid-treated as described for in situ hybridization. Antigen retrieval, a technique to improve the accessability of antibodies to target structures, is indispensable for satisfactory results of immunohistochemical staining. To remove unspecific binding a blocking step with antibody diluent or goat serum is carried out. Results are visualized by addition of BCIP and NBT for AP-conjugated antibodies (Burnett et al., 1997). Aims of the study The fundamental object of my study was to confirm results from previous microarray hybridizations, and to find out if maternal behavior was accompanied by induction of Arc using in situ hybridization, immunohistochemistry and qRT-PCR. 14 Results Papanicolaou tests Before using the main methods for detection and analysis of Arc, new samples of brain from mice having undergone behavior tests had to be obtained. Prior to the behavior tests the hormonal stage of each female mouse had to be determined. Hormone levels are low in diestrus. Papanicolaou staining is a medical method for detection of infections or cancer of the genital tract, but also for determination of sex cycle. By sampling cells from the vagina and using different dyes, the special staining of cells can be analyzed. Papanicolaou tests were performed with vaginal smears to check stage of estrus cycle. Phases with specific patterns and staining of different cell types were observed (Figure 3). Mice in diestrus (Figure 3A) were chosen for behavior tests to keep hormonal influence as low as possible. A B C Figure 3. Papanicolaou tests showing specific staining pattern of different hormonal phases. A: Smear of mouse in diestrus. Mostly leukocytes, stained darkblue, and nucleated cells are noted in diestrus. B: Smear of mouse in estrus. Estrus is characterized by irregularly shaped, large, epithelial cells without nuclei, which appear pink and orange. C: Smear of mouse in postestrus. In postestrus, also called metestrus, still large epithelial cells are visible, but they are accompagnied by invading leukocytes. Pictures are taken with ProgRes C14 (Jenoptik, Jena, Germany) at light microscope (Leica). Behavior tests Behavior tests were carried out during night with 10 female virgin mice. All of them showed perfect maternal behavior to 3 pups in the cage. They retrieved the pups succcessfully to one corner, started building a nest and licking the young. This behavior was usually completed after 8-10 minutes. Quantification of Arc mRNA in whole mouse brain To assess the amount of Arc transcripts in the whole brain of female mice that have been killed at different time points after exposure to pups qRT-PCR was carried out. Twelve brains taken out after different periods of time (2x 0 min, 3x 10 min, 3x 30 min, 2x 60 min and 2x 120 min) were tested for Arc expression in comparison to expression of Tubulin. The melting curve showed just one peak, assuring that the highest amount of double strands was the DNA I want to measure. The results showed inconsistent expression of Arc. RNA values in brains from same time points varied widely, especially in brains of females, which 15 had been killed after 10 min, 30 min and 120 min of pup exposure. In those samples up to 200% difference of mRNA value could be noted. Gene transcription was low at 0 min, comprising values of 0.396 and 0.234 compared to Tubulin. The mean value peaked at 120 min with a ratio of 0.865 (Table 1). Table 1. Expression values for Arc compared to Tubulin. set 3 mean value time point set 1 set 2 0 min 0.396 0.234 0.315 0.67 10 min 0.366 0.707 0.581 0.587 30 min 0.819 0.321 0.576 60 min 0.48 0.572 0.526 120 min 1.18 0.55 0.865 Table shows RNA values of 12 virgin mice killed at different time points after exposure to pups. The mean value was calculated from value of set 1, set 2 and value of set 3. Arc gene expression in mouse brain Arc expression was analyzed by in situ hybridization. Five different riboprobes were designed with help of five different pairs of primers (Table 2). Labeled antisense probes as well as sense probes were applied to perfused brain sections from different time points. The slides showed no differences in staining pattern with antisense and sense probes. Slight differences could be found in intensity of staining, but no obvious or significant signals can be detected. B A Figure 4. In situ hybridization with Arc-1 (Table 2) on perfused brain sections of 1 h after exposure to pups. A: Antisense B: Sense. Dark spots are due to development of bubbles during brain sectioning. Pictures were taken with Camera ProgRes C14 (Jenoptik) at light-optical microscope (Leica). A B Figure 5. In situ hybridization with Arc-2 (Table 2) on perfused brain sections of 30 min after exposure to pups. A: Antisense. B: Sense. Dark spots are due to development of bubbles during brain sectioning. Pictures were taken with Camera ProgRes C14 (Jenoptik) at light-optical microscope (Leica). 16 A B Figure 6. In situ hybridization with Arc B (Table 2) on perfused brain sections of 10 min after exposure to pups. A: Antisense B: Sense. Dark spots are due to development of bubbles during brain sectioning. Pictures were taken with Camera ProgRes C14 (Jenoptik) at light-optical microscope (Leica). A B Figure 7. In situ hybridization with Arc C (Table 2) on perfused brain sections of 10 min after exposure to pups. A: Antisense B: Sense. Dark spots are due to development of bubbles during brain sectioning. Pictures were taken with Camera ProgRes C14 (Jenoptik) at light-optical microscope (Leica). A B Figure 8. In situ hybridization with Arc D (Table 2) on perfused brain sections of 10 min after exposure to pups. A: Antisense B: Sense. Dark spots are due to development of bubbles during brain sectioning. Pictures were taken with Camera ProgRes C14 (Jenoptik) at light-optical microscope (Leica). ARC protein localization in mouse brain To assess expression of ARC immunohistochemical staining with anti-Arc was carried out on brains of virgin mice at 0 min, 10 min, 30 min, 60 min and 120 min after exposure to pups. Fluorescein isothiocyanate (FITC) staining (Figure 9) detected ARC expression at all times distributed in different parts of sagittal halves of brain. For a better overview, I additionally performed antibody staining with AP (Figures 10, 11). In those brain sections as well I found wide spread occurrence of ARC. Very strong signals were found in the MPOA, cortex region and brainstem (Figures 10, 11). Staining with FITC as well as AP was mainly noted in cell nuclei, suggesting translation of Arc mRNA in this area. In different brain sections strong staining of fibers could be seen (Figure 12). This points to the role of ARC as a cytoskeletal protein that binds to F-ACTIN at the dendrites. 17 Comparison of protein expression in different brain regions revealed most intense ARC occurrence in brains of females that had been killed after 30 min of pup exposure (Figure 10D, 11E, 11F). Brains that had been dissected 10 min and 2 h past such exposure showed weak ARC expression (Figure 11D, 11I), but same type of pattern (10C, 10F). Control sections incubated overnight with antibody dilutent instead of first antibody revealed no fluorescent signals (Figure 10A, 11A). A B Figure 9. Cutout of MPOA region in virgin mouse brain 30 min after exposure to foreign offspring A: Nuclei were counterstained with 4',6-Diamidino-2-phenylindol (DAPI); magnification 400x. B: Staining with FITC; magnification 400x. Pictures were taken with camera (Leica) at fluorescence microscope (Leica DMRXE). A B C D E F Figure 10. Mouse brain of different time points after exposure to foreign offspring; AP staining A: Control section without application of anti-ARC antibody. B: 0 min without exposure C: 10 min after exposure to foreign offspring D: 30 min after exposure to foreign offspring E: 1 h after exposure F: 2 h after exposure to foreign offspring. 18 A C B E F H I D G Figure 11. Cutouts of mouse brain of different time points after exposure to foreign offspring; AP staining A: Cortex region of control section without application of Anti-ARC antibody B: 0 min without exposure to foreign offspring staining in cortex C: 0 min without exposure to foreign offspring staining in MPOA D: 10 min after exposure to foreign offspring weak staining signals in cortex E: 30 min after exposure to foreign offspring most intensive staining in cortex F: 30 min after exposure to foreign offspring staining in MPOA G: 1 h after exposure to foreign offspring intensive staining in cortex H: 1 h after exposure to foreign offspring intensive staining in and MPOA I: 2h after exposure to foreign offspring weak staining in cortex. A B Figure 12. Cutouts of mouse brain of different time points after exposure to foreign offspring; FITC staining; magnification 400x; light exposure time 3.5s. A: 60 min after exposure to foreign offspring B: 30 min after exposure to foreign offspring. 19 Discussion Maternal behavior induces Arc Several studies have shown that Arc is involved in processing information and that it is increased in situations of high neural activity (Lyford et al., 1995; Link et al., 1995). As Arc was also found differentially expressed in mice exposed to pups compared to mice without exposure (Shi et al., 2005), I suspected Arc mRNA to be induced by maternal behavior. Quantitative RT-PCR was performed to test this hypothesis. RNA values in brains from the same time point varied widely, especially in brains of females, which had been killed after 10 min, 30 min and 120 min of pup exposure. So no firm conclusion can be drawn from those results. However, the combined data showed slow increase of Arc mRNA from 0 min to 120 min after pup exposure in mouse brain after exposure to foreign offspring. This suggests an Arc - inducing role for maternal care, though previous studies have reported Arc mRNA reaching highest levels 30 – 60 min after induction (Nishimura et al., 2003). In situ hybridization experiments My goal was to examine RNA expression of the Arc gene by performing in situ hybridization. However, no differences were found in staining pattern with antisense and sense probes, though there have been reported various successful experiments of in situ hybridization to Arc mRNA (Nishimura et al., 2003; Li et al., 2005; Rosi et al., 2005). I was not able to detect any significant signals in the brain sections even with riboprobes identical to those described in the papers (Nishimura et al., 2003; Li et al., 2005). Reasons for this discrepancy can be multifarious. One possibility is that the probes did not bind specifically, so that binding all over the whole brain caused unspecific signals and high background. Also, the brain sections were of bad quality due to perfusion of the brains before sectioning. Unclear role for ARC in maternal care Constitutive protein expression has been reported before in hippocampus, amygdala, hypothalamus, striatum and cortex (Lyford et al., 1995; Link et al., 1995). I have confirmed findings in cortex by fluorescence and immunohistochemical AP staining of brains without exposure to pups at time point 0 min. Additionally I have found ARC protein in the MPOA and brainstem leading me to the conclusion that ARC is also constitutively present in those areas. ARC was first described as a cytoskeletal protein binding to F-ACTIN and localizing at dendrites (Lyford et al., 1995). I was able to confirm those observations as ARC staining was found not only in cell nuclei, but also at nerve fibers detected as thin long lines in MPOA and brainstem. As expected, ARC was discovered in MPOA, a very important region for maternal behavior, which supports the idea of ARC being involved in maternal care. Countering this hypothesis is the fact that I was never able to detect ARC in the olfactory bulb, which represents the main sensory system for uptake of smells and recognition of pups. To find out if ARC is synthesized at higher than basal levels in the brain of virgin mice after exposure to pups, I analyzed protein patterns at different times after such exposure. None of the results were significant. So the role for ARC in maternal behavior still remains unclear. Unexplainable is the fact that basal levels of ARC was found in the brain at time 0 but not in sections obtained 120 min later. Either time 0 did not represent the constitutive level of ARC protein or another compound induced by maternal behavior caused a decrease of ARC after about two hours. 20 Reflection Why were the results of my investigation different from those previously obtained? One possible explanation comes from the fact that I used red light during the behavior tests and normal light shortly before killing the mice. Arc plays a role in the shift of light/dark cycle and can be induced by exposure to 600 lux fluorescent light (Nishimura et al., 2003). If red light can lead to high expression of Arc still remains to be tested, but the short exposure to normal room lights might have induced Arc and thus disturbed my results. As function, appearance and interactions of Arc gene, Arc transcript and ARC protein are not yet fully understood, I also cannot exclude other disturbing factors in my experiments. I do not know if possible sounds, odors or mouse interactions in the mouse house affect the induction of Arc. Aditionally, it is possible that previous experiments (Shi et al., 2005) have been interpreted incorrectly. Even though Arc was found differentially expressed in mice exposed to pups compared to mice without exposure (Shi et al., 2005), it cannot be concluded that increase of Arc mRNA is due to pup exposure. Possible future work To produce significant results it is inevitable to perform more experiments. Especially for the outcomes of the qRT-PCR, which varied widely, it is necessary to collect more samples at each time point to secure findings. Rearranging and adapting experimental procedure to new aspects will hopefully improve results. 21 Materials and Methods Laboratory animals All experiments with mice were conducted according to the guidelines of Uppsala Universitet. Behavior tests were performed with 19 virgin females from the inbred strain C57BL/6 from B&K Stockholm, Sweden. The pups that were presented to the adult mice were taken from any strain and had reached an age of 3-5 days at time of the tests. All mice were housed under similar circumstances in the mouse house. Bacteria For transformation and reproduction of plasmids I have used XL1-Blue Competent Cells of Escherichia coli (Stratagene). Primers Table 2. Primers4 for in situ hybridization used to design riboprobe and for qRT-PCR (direction 5’3’) Sequence1 name forward primer reverse primer 2 Arc-1 CCTACATGGGTTCCAAGACACTTT CCTGGTCTATT bp 2241-2764 Arc-23 CTACCGTCTGGAGAGGTGGG GGAACTGGCGAGTGGTTC bp 577-1082 Arc B TCCCAGATCCAGAACCACAT TATTCAGGCTGGGTCCTGTC bp 916-1388 Arc C CAGGGCTCTTTGGGTAATC GCTGGCTTGTCTTCACCTTC bp 2716-3357 Arc D GTGAAGACAAGCCAGCATGA CTCCAGGGTCTCCCTAGTCC bp 2344-2925 Q-Arc TACCCCTCATCTGTCTGCC GCCTACTTTTTGTTGCCTTTC Tubb3-A CGCCTTTGGACACCTATTCA ACTCTTTCCGCACGACATCT 1 GeneID: 54323 on NCBI 2 Nishimura et al., 2003 3 Li et al., 2005 4 http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi Papanicolaou tests In the afternoon before the behavior tests with exposure to pups, the stage of estrus cycle was determined. This was accomplished by taking vaginal smears of female C57BL/6 mice. The vagina of each mouse was rinsed with PBS using a glass pipette and the vaginal fluid was applied to microscope slides. Vaginal smears were stained with Papanicolaou (Merck) (Table 3). Diestrus was determined according to specific staining pattern of cells. Table 3. Papanicolaou (Merck, Darmstadt, Germany) stain for mice vaginal smear step solution time 1 95% ethanol rinse 2 H2O rinse 3 Harri`s haematoxylin 5 – 30 s 4 H2O rinse 5 95% Ethanol rinse 6 O.G.6 90 s 7 95% Ethanol rinse 8 EA 50 90 s 9 100% Ethanol rinse 22 Behavior tests with exposure to pups Behavior tests were carried out at night. Three alien pups were put into different corners of the cage with the chosen adult mouse (Figure 13). The behavior of the adult mouse was recorded for 15 min. In case of attacks against the young, the behavior test was interrupted. Maternal performance of the virgins included sniffing and licking the pups as well as retrieval and nest building. After the behavior tests the pups were removed from the cage and the female mice were killed by suffocation with CO2 after different times. Control mice were killed without exposure to pups. Figure 13. Virgin female mouse to be tested for maternal behavior with three pups in different corners of the cage (Shi et al., 2004). Treatment and fixation of tissues The brain was dissected out immediately, cut into sagittal halves and either frozen in tubes or imbedded in cryo medium (Tissue-Tek, Sakura Finetek Europe) on dry ice. Sections were stored in –80°C. Quantitative Reverse Transcription PCR RNA was extracted from 12 different brains of mice that had undergone behavior tests. Among those 12 brain samples time points 0 min, 60 min, 120 min were each covered twice and brain samples of time points 10 min and 30 min covered 3 times. The brains were homogenized in 3 ml TRIZOL reagent (Invitrogen Life Technologies) followed by incubation at room temperature for 5 min. After addition of 600 µl chloroform, centrifugation for 15 min at 500.000g was carried out at 4 – 8 °C resulting in phase separation. After taking the aqueous upper phase containing the RNA, 1.5 ml isopropyl alcohol was applied for precipitation of RNA and after 10 min incubation at room temperature centrifugation at same conditions as above was performed. The RNA pellet was washed with 75% ethanol, centrifuged at 500.000g for 5 min, dried and redissolved in 150 µl diethylpyrocarbonate (DEPC) (Sigma) treated water. 12 µl of the extracted RNA were treated with 2 µl Dnase (Promega) at 37°C for 30 min. 2 µl DNase stop solution at 65°C for 10 min ended enzymatic activity. After incubation at 70°C with 2 µl 10mM dNTPs and 2 µl 250 mM random primers, cDNA synthesis was performed by adding M–MLV-reverse transcriptase (Promega), RNase inhibitor (N211 B, Promega) and buffer (M531 A, Promega). Incubation at 42°C for 2 h completed cDNA synthesis. qRT-PCR was accomplished for the gene Arc using Tubulin as reference. 8 µl cDNA and 1 µl of each corresponding forward and reverse primer, Q-Arc and Tubb3-A (Table 2), were added to 10 µl SYBR Green Mix (Qiagen) containing the fluorescent dye as well as the HotStarTaq Polymerase. qRT-PCR was performed twice for each gene using the 12 different cDNA samples. A cycle (Table 4) was programmed on the rotor gene instrument (RG-3000, Corbett research) and a melting curve was established from 65°C to 95°C. 23 Table 4. QRT-PCR cycle operation denaturation annealing elongation temperature 95°C 57°C 72°C time 15 s 20 s 20 s Perfusion Five female mice, one for each time point, were perfused after behavior tests. Fixing by perfusion compared to fixing by submersion in fixative has been found to be much better at preserving the tissue and maintaining the RNA in good shape (Lawrence & Singer, 1985). Aditionally, background signals are reduced by removal of blood cells in tissue. After anesthesia of the mouse the chest was cut open and heart was excavated. The right atrium was punctured and the perfusion needle put into left ventricle. Perfusion was started with 30 – 50 ml heparin (5 U/ml), followed by 5 ml 5 % formalin. Needle was transferred into the right ventricle and perfusion completed with 10 – 20 ml 30 % sucrose. Subsequently the brain was dissected out and stored overnight in 30 % sucrose at 4°C. It was imbedded in cryo medium the next day and the sample stored at –80°C. Sectioning The imbedded brains were sectioned in a cryostat (Leica) at –18 to –20°C. The cryo blocks were cut into 12 - 20 µm thick sections and put onto microscope slides (Menzel-Gläser Super FrostPlus, Menzel GmbH & Co KG). Afterwards the slides were dried overnight at either room temperature or 37°C. The next day the slides were placed at -80°C for storage. In situ hybridization PCR was performed with various pairs of primers (Table 2) that were received from MWGBiotech AG. The PCR products were cut out of the gel and extracted with help of the QIAquick Gel extraction Kit (50) (Quiagen). 3 µl of the purified DNA were ligated with 1 µl T4 DNA Ligase (Promega) into the 1 µl T Easy Vector (Promega) (Figure 14). 5 µl 2x buffer for T4 DNA Ligase was used and the mix was incubated for 1 h at room temperature. Figure 14. Map of T Easy Vector. The map shows origin of plasmid, phage f1 replicating zone, the lac-operon, the multiple cloning region and possible places for digestion (pGEM-T and pGEM-T Easy Vector Systems, Technical Manual, Promega). 24 All ligation product was mixed with 50 µl Escherichia coli competent cell and 0.75 µl βmercaptoethanol. Transformation was induced by heatshock at 42°C for 45 s. Transformed bacteria were selected on agar plates made of 1L LB-medium (5 g yeast extract, 5 g NaCl and 10 g Bacto Tryptone per litre ) and 15 g Bacto agar with 1.6 ml X-Gal , 5 ml IPTG (both from Saveen Werner AB) and 1 ml ampicillin (Sigma) at 37°C overnight. A colony that had taken up the plasmid containing the Arc probe was grown at 37°C overnight in LB pH 7 with 0,1% ampicillin. The plasmid was purified with QIAspin Miniprep Kit (250) (Quiagen). The sense (T7 promoter) template was generated by digesting with 1 µl SalI (Biosciences Amersham, England) and the antisense (Sp6 promoter) template was generated by digesting with 1 µl SacII (New England Biolabs, MA, USA). Digestions were carried out with appropriate 10x buffer for 2 h at 37°C . The concentration of linearized product was determined by Nanodrop (ND-1000, Saveen Werner AB). To create digoxigenin-labeled RNA probes, RNA polymerases specific for the T7 and Sp6 promoters were used in a labeling mix (Table 5) for in vitro-transcription. Table 5. Labeling mix for in situ hybridization reagent volume DEPC H2O 10.5 µl Template DNA 2 µl Transcription buffer (Promega) 4 µl DIG-RNA labeling mix (Roche) 2 µl DTT 1 µl RNAse Inhibitor (Promega) 0.5 µl RNA-polymerase (Promega) 2 µl Total 20 µl concentration 1 µg/µl 5x 10x 250 mM 40 U/µl 20 U/µl The labeling mix (Table 5) was incubated for 2 h at 37°C. After incubation 2 µl of 0.2 M EDTA, 2.5 µl of 4 M LiCl and 300 µl 100% EtOH were added. The samples were incubated 30 min at –20°C and then centrifuged at 35.000g for 10 min at 4°C. After washing the pellet with EtOH and resuspension in 20 µl DEPC water, the labeled RNA probe was stored at – 80°C. Before hybridization brain section were fixed in 4% paraformaldehyde (PFA) (Sigma) and treated with different solutions (Table 6). Proteinase K buffer was made from 2.5 ml 1 M Tris-HCL pH 7.5 - 8, 250 µl EDTA and 5 µl Proteinase K (20 mg/ml) in 50 ml DEPC-treated H2O and acetylation buffer was made from 930 µl triethanolamine (Sigma), 110 µl 10 M NaOH and 125 µl acetic anhydride (Sigma) in 50 ml DEPC treated water. Table 6. Washing steps for tissue treatment step solution 1 1x PBS made of PBS tablets (Gibco) 2 4% PFA in 1x PBS 3 1x PBS 4 1x PBS 5 0.1 M HCl pH1 6 1x PBS 7 Proteinase K buffer 8 1x PBS 9 4% PFA in 1x PBS 10 1x PBS 11 Acetylation buffer 12 Add 125 µl Acetic Anhydride 13 1x PBS 25 time 5 min 20 min 5 min 5 min 10 min 5 min 5 min 5 min 15 min 5 min 5 min 5 min 5 min After denaturing the probe at 70°C and mixing it with the heated hybridization mix (Table 7), it was hybridized with digoxigenin-tagged DNA in a humid chamber overnight at 58 – 60°C. Table 7. Hybridization mix reagent Formamide (deionized) (Ambion, The RNA Company) 20x SSC (Sambrook et al., 1989) 50x Denharts (Sigma) tRNA (50 ng/µg) Sperm DNA (10 mg/ml) DEPC H2O Total amount 2 ml 1 ml 400 µl 20 µl 100 µl 480 µl 4 ml Various washing steps (Table 8) were performed to remove unspecific binding of labeled hybridization probe. Table 8. Washing step solution 1 5x SSC 2 0.2x SSC 3 1x RNA Wash (Sambrook et al. 1989) 4 1x RNA Wash/RNase A 5 1x RNA Wash 6 2x SSC 7 0.2x SSC 8 NT (Sambrook et al., 1989) 9 NT/1% blocking solution time 15 min 60 min 10 min 30 min 5 min 10 min 10 min 5 min 60 min temperature 53°C 53°C 37°C 37°C 37°C 37°C 37°C RT 37°C Polyclonal anti-digoxigenin antibody linked to AP (Roche Diagnostics) and diluted 1:1000 in NaCl-Tris buffer (NT) /milk powder blocking solution (50 ml NT buffer + 0.5 g blocking reagent (Roche Diagnostics)) was applied to brain tissue on object slides in humid chamber overnight at 4°C. After washing in NT and NaCl-Tris-MgCl2-Tween-buffer (NTMT) buffer (Table 9), slides were incubated at room temperature in staining solution containing BCIP and NBT (both Roche) between one and three hours or over night. Table 9. NTMT buffer reagent 5 M NaCl 1 M Tris-HCl pH 9.5 0.5 M MgCl2 Tween 20 (Sigma) Sterile H2O Tetramisole (Sigma) Total amount 2 ml 10 ml 10 ml 100 µl 78 ml 50 mg 100 ml The sections were washed with PBS and counterstained with 0.1% nuclear fast red solution (Sigma) for 30 s. To preserve the slides they were mounted with glycerol. 26 Immunohistochemistry Immunohistochemical staining with fluorescein isothiocyanate (FITC) was used to detect ARC protein in the brains of female mice. The tissue was fixed with 4% PFA and antigen retrieval with Target Retrieval Solution (Ready-to-Use, S1700, DakoCytomation) was performed at 95°C in a waterbath for 25 min. A solution of PBS containing 10% goat serum (S-1000, Vector Laboratories) and 0.1% Triton 100 X (Sigma) was applied at RT for 1 h. Brain sections were treated with diluted anti-ARC antibody (H-300, rabbit polyclonal IgG, Santa Cruz Biotechnology) (1:100) at 4°C overnight. The next day the slides were treated with secondary polyclonal Swine Anti-Rabbit Immunoglobulins/FITC antibodies (DakoCytomation) (1:20) at 4°C overnight. The sections were counterstained with 4',6diamidino-2-phenylindol (DAPI) (Vectashield Mounting Medium for fluorescence with DAPI, H-1200, Vector) and analyzed in the fluorescence microscope (Leica DMRXE). Between each step of processing, 3x 5 min washings with PBS or Tris Buffered Saline (TBS) made of 945 ml H2O, 25 ml 2 M Tris-base and 30 ml 5 M NaCl were performed. 27 Acknowledgements Reinald Fundele, thank you for giving me the opportunity to finish my studies in Uppsala, Sweden and providing me an insight into science. Yang Yu, thanks for teaching me everything I need to know and especially how to handle the mice. Umashankar Singh, tack så mycket for your guidance in the lab as well in the office. I appreciate very much talking with you. To all people in the department of development and genetics. I really liked to have every one of you around me, especially on cake thursdays and during world championship. Thanks for being there and cheering up lab work during my degree project. 28 References Bellmann H. 1995. Bienen, Wespen, Ameisen. Hautflügler Mitteleuropas. Franckh-Kosmos, Stuttgart. Brown J.R., Ye H., Bronson R.T., Dikkes P., Greenberg M.E. 1996. A Defect in Nurturing in Mice Lacking the Immediate Early Gene fosB. Cell, 86: 297–309. Burnett R., Guichard Y., Barale E. 1997. Immunohistochemistry for light microscopy in safety evaluation of therapeutic agents: an overview. Toxicology. 119: 83-93. Calamandrei G., Wilkinson L.S., Keverne E.B. 1992. Olfactory Recognition of Infants in Laboratory Mice: Role of Noradrenergic Mechanisms. Physiology & Behavior, 52: 901-907. Champagne F.A., Weaver I.C.G., Diorio J., Dymov S., Szyf M., Meaney M.J. 2006. Maternal Care Associated with Methylation of the Estrogen Receptor- 1b Promoter and Estrogen Receptor- Expression in the Medial Preoptic Area of Female Offspring. Endocrinology 147: 2909–2915. Collins L.L., Lee Y.-F., Heinlein C.A., Liu N.-C., Chen Y.-T., Shyr C.-R., Meshul C.K., Uno H., Platt K.A., Chang C. 2004. Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proceedings of the National Academy of Sciences, 101: 42, 15058-15063. Da Costa A.P., Broad K.D., Kendrick K.M. 1997. Olfactory Memory and Maternal Behaviour-induced Changes in c-fos and zif/268 mRNA Expression in the Sheep Brain. Brain Res. Mol. Brain Res., 46: 63-76. Donnelly M.A. 1989. Reproductive phenology and age structure of Dendrobates pumilio in northeastern Costa Rica. Journal of Herpetology 23: 362-367. Fatemi M., Hermann A., Gowher H., Jeltsch A. 2002. Dnmt3a and Dnmt1 functionally cooperate during de novo methylation of DNA. European Journal of Biochemistry, 269: 4981-4. Fosnaugh, J.S., Bhat R.V., Yamagata K., Worley P.F., Baraban J.M. 1995. Activation of arc, a Putative “Effector” Immediate Early Gene, by Cocaine in Rat Brain. Journal of Neurochemistry, 64: 2377-2380. Fox M.W., Coats C.D. 1989. Old MacDonald's Factory Farm: The Myth of the Traditional Farm and the Shocking Truth About Animal Suffering in Today's Agribusiness. Continuum International Pub Group. Franz J.R., Leo R.J., Steuer M.A., Kristal M.B. 1986. Effects of Hypothalamic Knife Cuts and Experience on Maternal Behavior in the Rat. Physiology & Behavior, 38: 629-640. Freude H., Harde K.W., Lohse G.A. 1971. Staphylinoidea; Fam. Silphidae, Catopidae, Liodidae, Scydmaenidae, Ptilidae, Scaphididae Die Käfer Mitteleuropas, Band 3: 190-201. 29 Gandelman R., Zarrow M. X., Denenberg V. H. 1970. Maternal behavior: Differences between mother and virgin mice as a function of the testing procedure. Developmental Psychobiology, 3: 207-214. Gandelmann R. and Vom Saal F. 1977. Exposure to Early Androgen Attenuates AndrogenInduced Pup-Killing in Male and Female Mice. Behavioral Biology, 20: 252-260. Gulledge C.C., Mann P.E., Bridges R.S., Bialos M., Hammer R.P. 2000. Expression of mopioid receptor mRNA in the medial preoptic area of juvenile rats. Developmental Brain Research 119: 269–276. Guzowski J.F., Lyford G.L., Stevenson G.D., Houston F.P., McGaugh J.L. Worley P.F., Barnes C.A. 2000. Inhibition of Activity-Dependent Arc Protein Expression in the Rat Hippocampus Impairs the Maintenance of Long-Term Potentiation and the Consolidation of Long-Term Memory. The Journal of Neuroscience, 20: 3993-4001. Hausfater G., Hrdy S.B. 1984. Infanticide: Comparative and Evolutionary Perspectives. New York, Aldine. Hendrich B., Guy J., Ramsahoye B., Wilson V.A., Bird A. 2001. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes & Dev., 15: 710-723. Higuchi R., Dollinger G., Walsh P. S., Griffith R. 1992. Simultaneous amplification and detection of specific DNA sequences. Biotechnology 10: 413-417. Insel T.R., Young L.J. 2001. The neurobiology of attachment. Nature Reviews Neuroscience 2: 129-136. Jin L., Lloyd R.V. 1997. In situ hybridization: methods and applications. Journal of Clinical Laboratory Analysis. 11: 2-9. Kelly M.P. and Deadwyler S.A. 2002. Acquisition of a Novel Behavior Induced Higher Levels of Arc mRNA than does Overtrained Performance. Neuroscience, 110: 617-626. Kendrick K.M., Da Costa, A.P.C., Broad K.D., Ohkura S., Guevara R., Lévy F., Keverne E.B. 1997. Neural Control of Maternal Behaviour and Olfactory Recognition of Offspring. Brain Research Bulletin, 44: 4, 383–395. Keverne E.B. 1988. Central Mechanisms Underlying the Neural and Neuroendocrine Determinants of Maternal Behaviour. Psychoneuroendocrinology. 13: 127-141. Kuzmin S., Ishchenko V., Tuniyev B., Beebee T., Andreone F., Nyström P., Anthony B., Schmidt B., Ogrodowczyk A., Ogielska M., Bosch J., Miaud C., Loman J., Cogalniceanu D., Kovács T., Kiss I., Puky M., Vörös J., Martínez-Solano I., Salvador A., García-París M. and Gil E.R. 2004. Rana temporaria. 2006 IUCN Red List of Threatened Species. Lanahan A. and Worley P. 1998. Immediate-Early Genes and Synaptic Function. Neurobiology of Learning and Memory, 70: 37-43. 30 Lawrence J.B., Singer R.H. 1985. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nuc Acids Res 15:1777-1799. Lefebvre L., Viville S., Barton S. C., Ishino F., Keverne E. B., Surani M. A. 1998. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nature Genetics, 20: 163-169. Lehninger A.L., Nelson D.L., Cox M.M. 2001. Biochemie. Springer Verlag, Berlin. Leitsch A.R., Schwarzacher T., Jackson D., Leitsch I.J. 1994. In situ-Hybridisierung, Spektrum Akademischer Verlag Heidelberg, Berlin. Lévy F., Keller M., Poindron P. 2004. Olfactory regulation of maternal behavior in mammals. Hormones and Behavior 46: 284-302. Li L.-L., Keverne E. B., Aparicio S. A., Ishino F., Barton S. C., Surani M. A. 1999. Regulation of Maternal Behavior and Offspring Growth by Paternally Expressed Peg3. Science, 284: 330-333. Li L., Carter J., Goa X., Whitehead J., Tourtellotte W.G. 2005. The NeuroplasticityAssociated Arc Gene Is a Direct Transcriptional Target of Early Growth Response (Egr) Transcription Factors. Molecular and Cellular Biology, 25: 10286-10300. Link W., Konietzko U., Kauselmann G., Krug M., Schwanke B., Frey U., Kuhl D. 1995. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA, Neurobiology, 92: 5734-5738. Liu D., Bei D., Parmar H., Matus A. 2000. Activity-regulated, cytoskeleton-associated protein (Arc) is essential for visceral endoderm organization during early embryogenesis. Mechanisms of Development, 92: 207-215. Lonstein J.S., De Vries G.J. 2000. Sex differences in the parental behavior of rodents Neuroscience & Biobehavioral Reviews. 24: 669-686. Lovic V., Gonzalez A., Fleming A.S. 2001. Effects of early life experiences on adult maternal behavior. Psychobiol. 39: 19-33. Lucas B.K., Ormandy C.J., Binart N., Bridges R.S., Kelly P.A. 1998. Null Mutation of the Prolactin Receptor Gene Produces a Defect in Maternal Behavior. Endocrinology, 139: 10, 4102-4107. Lyford G.L., Yamagata K., Kaufmann W.E., Barnes C.A., Sanders L.K., Copeland N.G., Gilbert D.J., Jenkins N.A., Lanahan A. and Worley P. 1995. Arc, a Growth Factor and Activity-Regulated Gene, Encodes a Novel Skeleton-Associated Protein That Is Enriched in Neuronal Dendrites. Neuron, 14: 433-445. 31 Mark L. P., Daniels D. L., Naidich T. P., Hendrix L. E. 1995. Limbic Connections. American Journal of Neuroradiology, 16: 1303-1306. McCarthy M. and Vom Saal F. 1985. The Influence of Reproductive State On Infanticide by Wild Female House Mice (Mus musculus). Physiology & Behavior, 35: 843-849. McCarthy M., Bare J.E., Vom Saal F. 1986. Infanticide and Parental Behavior in Wild Female House Mice: Effects of Ovariectomy, Adrenalectomy and Administration of Oxytocin and Prostaglandin F2α. Physiology & Behavior, 36: 17-23. Milius S. 1998. Infanticide Reported in Dolphins. Science News, 154: 36. Müller H.J., Röder T. 2004. Der Experimentator: Microarrays. Spektrum Akademischer Verlag, Heidelberg. Nishimura M., Yamagata K., Sugiura H. 2003. The Activity-Regulated CytoskeletalAssociated (Arc) Gene is a new Light-Inducable Early Gene in the mouse Suprachiasmatic Nucleus. Neuroscience, 116: 1141-1147. Numan M. 1988. Neural Basis of Maternal Behaviour in the Rat. Psychoneuroendocrinology, 13: 47-62. Nyberg F., Sanderson K., Glamsta E.L. 1997. The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers. 43: 147-56. Ogawa S., Eng V., Taylor J., Lubahn D.B., Korach K.S., Pfaff D.W. 1998. Roles of Estrogen Receptor-a Gene Expression in Reproduction-Related Behaviors in Female Mice Endocrinology, 139: 5070-5081. Ons S., Martí O., Armario A. 2004. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. Journal of Neurochemistry, 89: 1111–1118. Ormandy C.J., Camus A., Barra J., Damotte D., Lucas B., Buteau H., Edery M., Brousse N., Babinet C., Binart N., Kelly P.A. 1997. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & Development, 11: 167-178. Pinaud R., Penner M.R., Robertson H.A. Curriea R.W. 2001. Upregulation of the immediate early gene arc in the brains of rats exposed to environmental enrichment: implications for molecular plasticity. Molecular Brain Research, 91: 50-56. Poindron P., Lévy F., Krehbiel D. 1988. Genital, Olfactory, and Endocrine Interactions in the Development of Maternal Behaviour in the Parturient Ewe. Psychoneuroendocrinology, 13: 99-125. Polis G.A. 1990. The Biology of Scorpions. Stanford University Press, California. Populin R. and D'Amato F.R. 1987. Mother-Offspring Interaction and Pup Development in Genetically Deaf Mice. Behavior Genetics, 17: 465-475. 32 Pough F. H., Andrews R. M., Cadle J. E., Crump M., Savitsky A. H., Wells K. D. 2003. Herpetology (3. Auflage). Pearson Prentice Hall, Upper Saddle River, New Jersey. Pough H., Janis C., Heiser J. 2005. Vertebrate Life. Pearson Prentice Hall, Upper Saddle River, New Jersey. Rodgers W. 1999. Animals in Belgrade zoo also feel effects of war. CNN.com Rosenblatt J.S., Mayer A.D., Giordano A.L. 1988. Hormonal Basis during Pregnancy for the Onset of Maternal Behaviour in the Rat. Psychoneuroendocrinology, 13: 29-46. Rosi S., Ramirez-Amaya V., Vazdarjanova A., Worley P. F., Barnes C. A., Wenk G. 2005. Neuroinflammation Alters the Hippocampal Pattern of Behaviorally Induced Arc Expression. The Journal of Neuroscience, 25: 723-731. Rossant J. and Cross. J.C. 2001. Placental development: Lessons from mouse mutants. Nature Reviews Genetics 2: 538-548. Sambrook J., Fritsch E.F., Maniatis T. 1989. Molecular Cloning, A Laboratory Manual, Second Edition. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, N.Y. Sheng M. and Greenberg M.E. 1990. The Regulation and Function of c-fos and Other Immediate Early Genes in the Nervous System. Neuron, 4: 477-485. Shi W., Lefebvre L., Yu Y., Otto S., Krella A., Orth A., Fundele R. 2004. Loss-of-imprinting of Peg1 in mouse interspecies hybrids is correlated with altered growth. Genesis, 39: 65-72. Shi, W. 2005. Growth and Behaviour. Epigenetic and Genetic Factors Involved in Hybrid Dysgenesis. Acta Universitatis Upsaliensis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 11.51 pp. Uppsala. ISBN 91-5546147-6 Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C., Cross J.C., Weiss S. 2003. Pregnancy-Stimulated Neurogenesis in the Adult Female Forebrain Mediated by Prolactin. Science, 299: 117-120. Simantov R., Snyder H. 1976. Morphine-like peptides in mammalian brain: Isolation, structure elucidation, and interactions with the opiate receptor. Proceedings of the National Academy of Sciences, 73: 2515. Steward O. and Worley P.F. 2001. Selective Targeting of Newly Synthesized Arc mRNA to Active Synapses Requires NMDA Receptor Activation. Neuron, 30: 227-240. Tan A., Moratalla R., Lyford G.L., Worley P., Graybiel A.M. 2000. The Activity Regulated Cytoskeletal-Associated Protein Arc Is Expressed in Different Striosome–Matrix Patterns Following Exposure to Amphetamine and Cocaine. Journal of Neurochemistry, 74: 20742077. 33 Thomas S.T. and Palmiter R.D. 1997. Impaired Maternal Behavior in Mice Lacking Norepinephrine and Epinephrine the dopamine b-hydroxylase (Dbh) gene. Cell, 91: 583-592. Vogt C. 1842. Untersuchungen über die Entwicklungsgeschichte der Geburtshelferkröte (Alytes obstetricians). Solothurn: Jent und Gassmann Weaver I.C.G., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. 2004. Epigenetic programming by maternal behavior. Nature Neuroscience, 7: 847-854. Wehner R., Gehring W. 1995. Zoologie. Georg Thieme Verlag, Stuttgart Wehr R., Mansouri A., de Maeyer T., Gruss P. 1997. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development, 124: 4447-4456. Wettschureck N., Moers A., Hamalainen T., Lemberger T., Schuetz G., Offermanns S. 2004. Heterotrimeric G Proteins of the Gq/11 Family Are Crucial for the Induction of Maternal Behavior in Mice. Molecular and Cellular Biology, 24: 8048-8054. 34