* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download chemistryandmacromolecules3
Signal transduction wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene expression wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Western blot wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Interactome wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Biosynthesis wikipedia , lookup
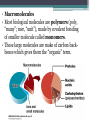
UNIT 1 BIOCHEMISTRY Part 3 Hillis Textbook Chapter 2-3 Macromolecules (Building Blocks of Life) 2007-2008 MACROMOLECULES OF LIFE • BIOMOLECULES – macromolecules essential for living things to survive. • Heterotrophs get biomolecules from the food that they consume (typically). This is why eating food is critical to survival. • Autotrophs can produce them on their own by special processes (ex. Photosynthesis). • Macromolecules • Most biological molecules are polymers (poly, “many”; mer, “unit”), made by covalent bonding of smaller molecule called monomers. • These large molecules are make of carbon backbones which gives them the “organic” term. Remember why carbon is so important? •Carbon has FOUR electrons capable of sharing, which means it can form FOUR covalent bonds! CARBON EVERYWHERE! •Biomolecules are made of these strong bonds with carbon as a central atom. Polymers are formed and broken apart in reactions involving water. • Condensation Reaction or Dehydration synthesis —removal of water links monomers together. Requires enzymes and energy input. REMOVE WATER TO BUILD! MONOMER TO POLYMER • Hydrolysis—addition of water breaks a polymer into monomers. Requires enzymes and releases energy. ADD WATER TO BREAK! POLYMER TO MONOMER Carbohydrates Cn ( H 2O) n • formed by linking similar sugar monomers (monosaccharides) to form polysaccharides • Source quick energy use and “first choice” storage of energy. • Recognition or signaling molecules that can trigger specific biological responses Monosaccharides are simple sugars. Pentoses are 5-carbon sugars Ribose and deoxyribose are the backbones of RNA and DNA. Hexoses (C6H12O6) include glucose, fructose and galactose. • Monosaccharides are covalently bonded by condensation reactions that form glycosidic linkages. • Sucrose is a disaccharide. • Oligosaccharides are small polysaccharides (3-6 sugars) • Many have additional functional groups. • They are often bonded to proteins and lipids on cell surfaces, where they serve as recognition signals (they stick out of the cell membrane like street signs). • Polysaccharides are large polymers of monosaccharides; the chains can be branching. • Starches—a branched family of polysaccharides of glucose found in plants • Glycogen—highly branched polymer of glucose; main energy storage molecule in mammals (found in the liver cells). • Cellulose—the most abundant carbon-containing (organic) biological compound on Earth; stable and unbranched; good structural material Lipids • formed by interactions of lipid monomers, such as fatty acids and glycerol backbones. • Contain hydrocarbons (composed of C and H atoms); they are insoluble in water because of many nonpolar covalent bonds. • When close together, weak but additive van der Waals interactions hold them together. Store energy in C—C and C—H bonds • Play structural role in cell membranes • Fat in animal bodies serves as thermal insulation Triglycerides (simple lipids) • Fats—solid at room temperature • Oils—liquid at room temperature • They have very little polarity and are extremely hydrophobic. Triglycerides consist of: • Three fatty acids— nonpolar hydrocarbon chain attached to a polar carboxyl (—COOH) (carboxylic acid) • One glycerol—an alcohol with 3 hydroxyl (—OH) groups • Synthesis of a triglyceride involves three condensation reactions. • Fatty acid chains can vary in length and structure. • In saturated fatty acids, all bonds between carbon atoms are single; they are saturated with hydrogens. • In unsaturated fatty acids, hydrocarbon chains contain one or more double bonds. These acids cause kinks in the chain and prevent molecules from packing together tightly. Amphipathic: the lipid has a hydrophilic end and a hydrophobic tail. Phospholipid—two fatty acids and a phosphate compound bound to glycerol The phosphate group has a negative charge, making that part of the molecule hydrophilic. • In an aqueous environment, phospholipids form a bilayer. • The nonpolar, hydrophobic “tails” pack together and the phosphatecontaining “heads” face outward, where they interact with water. • Biological cell membranes have this structure: PHOSPHOLIPID BILAYER Nucleic acids • from four kinds of nucleotide monomers • polymers are specialized for storage, transmission, and use of genetic information. DNA = deoxyribonucleic acid RNA = ribonucleic acid Nucleotide: Pentose sugar + N-containing base + phosphate group Bases: • Pyrimidines—single rings • Purines—double rings Sugars: • DNA contains deoxyribose • RNA contains ribose • Nucleotides bond in condensation reactions to form phosphodiester linkages. • Nucleic acids grow in the 5′ to 3′ direction. Function: Protein synthesis Function: Genetic Code Proteins • Formed from different combinations of 20 amino acid monomers • Amino and carboxylic acid functional groups allow them to act as both acid and base. • The R group differs in each amino acid. • They are grouped according to properties conferred by the R groups. Major functions of proteins: • Enzymes—catalytic proteins • Defensive proteins (e.g., antibodies) • Hormonal and regulatory proteins—control physiological processes • Receptor proteins—receive and respond to molecular signals • Storage proteins store amino acids • Structural proteins—physical stability and movement • Transport proteins carry substances (e.g., hemoglobin) • Genetic regulatory proteins regulate when, how, and to what extent a gene is expressed • Amino acids are linked in condensation reactions to form peptide linkages or bonds. • Polymerization takes place in the amino to carboxyl direction. • • • • • • Polypeptides or proteins range in size from insulin, which has 51 amino acids, to huge molecules such as the muscle protein titin, with 34,350 amino acids. Primary structure of a protein—the sequence of amino acids Secondary structure—regular, repeated spatial patterns in different regions, resulting from hydrogen bonding • α (alpha) helix—right-handed coil • β (beta) pleated sheet—two or more polypeptide chains are extended and aligned Tertiary structure—polypeptide chain is bent and folded; results in the definitive 3-D shape. The outer surfaces present functional groups that can interact with other molecules. Interactions between R groups determine tertiary structure. • Disulfide bridges hold a folded polypeptide together (sulfur bonds) • Ionic interactions form salt bridges •Hydrophobic side chains can aggregate •van der Waals interactions between hydrophobic side chains •Hydrogen bonds stabilize folds Secondary and tertiary protein structure derive from primary structure. Denaturing—heat or chemicals are used to disrupt weaker interactions in a protein, destroying secondary and tertiary structure. The protein can return to normal when cooled—all the information needed to specify the unique shape is contained in the primary structure. • Quaternary structure—two or more polypeptide chains (subunits) bind together by hydrophobic and ionic interactions, and hydrogen bonds. • These weak interactions allow small changes that aid in the protein’s function. Factors that can disrupt the interactions that determine protein structure (denaturing): •Temperature •Concentration of H+ •High concentrations of polar substances •Nonpolar substances