* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The title: A Structure for Deoxyribose Nucleic Acid
Comparative genomic hybridization wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Community fingerprinting wikipedia , lookup
Molecular evolution wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Non-coding DNA wikipedia , lookup
Holliday junction wikipedia , lookup
Molecular cloning wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Maurice Wilkins wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
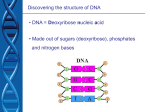
J. D. WATSON and F. H. C. CRICK A Structure for Deoxyribose Nucleic Acid Nature, 2 April 1953, VOL 171,737 1953 Commentary by Tom Zinnen The paragon of elegance, this paper is renowned for its simplicity, clarity, durability and understatement. The four key characteristics of this model of DNA endure: DNA is double-stranded, anti-parallel, complementary, and the double strands are in a double-helix. And because the authors were 25 and 35 years old, respectively, when the paper was published, it serves as an example to students that sometimes, extraordinary discoveries are made by people within a few years of completing high school. In the early 1950's, the structure of DNA had become a crucial puzzle, following the discovery that DNA and not protein was the "transforming" principle. The puzzle was more intriguing because of the challenge of figuring out how a polymer composed of only 4 different "letters" could encode for a polymer such as proteins that are composed of 20 different letters. The date: 2 April 1953. (Note: Also the year of the Hershey-Chase experiment that tested whether DNA or protein was the informational molecule.) The title: A Structure for Deoxyribose Nucleic Acid Note the use of "deoxyribose nucleic acid" rather than "deoxyribonucleic acid" that is more commonly used today. Note that this is "a" structure and not "the" structure. Other possible configurations were not excluded. And in fact, known configurations of DNA include single-stranded and triple-stranded, and even the double-stranded type occurs in three forms (A, B and Z; they describe the B form). Lead sentence: "We wish to suggest a structure for the salt of deoxyribose nucleic acid (D.N.A.)." DNA is an acid, owing to the phosphate groups between each deoxyribose. The "salt" of DNA is the form in which some of the hydrogen ions have disassociated from the phosphate group. The salt then has a net negative charge, specifically at the oxygens of the phosphates. This net negative charge is a key characteristic to keep in mind when reflecting on the function of histones or on the movement of DNA in electrophoresis. The Pauling-Corey model: "Their model consists of three intertwined chains, with the phosphates near the fibre axis, and the bases on the outside." Note that about the only characteristic in common with the Watson-Crick model is "twining." The differences include three strands instead of two, the sugar-phosphate backbone is in the center rather than on the outside, and there is no mention of base-pairing. Without base-pairing, there is no explanation for "Chargaff's Rules": in DNA the moles of A and T are about the same, as are the moles of G and C. Note that in 1952 "Chargaff's Rules" referred only to the amounts of A and T, and of G and C. The idea that A binds to T and T to A, and G binds to C and C to G, is first presented in this paper. Yet note that the two specific criticisms that Watson and Crick list about the Pauling-Corey model center on net charge and on distances between atoms. Perhaps these were sufficient to show the model was unsatisfactory. The Watson-Crick Model "This structure has two helical chains each coiled round the same axis (see diagram)." See diagram, indeed. Further evidence that 1 picture = 1kilowords. Characteristics 1 and 2: DNA is double-stranded in a double-helix. Note that two helical chains can share a common axis without necessarily being linked the way DNA is. The rung-and-ladder scheme is so commonplace now that we may not realize that other double-helix arrangements are plausible. Biochemistry review time: "We have made the usual chemical assumptions, namely, that each chain consists of phosphate diester groups joining ß-D-deoxyribofuranose residues with 3',5' linkages." Phosphate diester: This refers to a single phosphate that is linked by ester-type bonds to two nucleosides. It is a diester because there are two ester linkages, one to each nucleoside. ß-D-deoxyribofuranose: organic chemistry or biochemistry. 1. ribo refers to ribose, a sugar with 5 carbons. 2. deoxyribo refers to deoxyribose, a ribose that's missing an oxygen at the Number 2 carbon. 3. deoxyribofuranose means a particular five-carbon sugar that's in a circle with five members (Carbons 1 through 4 and an oxygen), rather than in a linear arrangement. Carbon 5 sticks up above the five-member circle. 4. ß means that of the two possible arrangements of the deoxyribofuranose, this molecule is in the version in which both the hydroxyl group of the Number 1 carbon and the Number 5 carbon are both above the plane of the circular furanose. The Taken from: http://www.accessexcellence.org/RC/AB/BC/casestudy2.php 5. 6. alternative is the alpha arrangement, in which the hydroxyl group would be below the plane of the circular furanose, while the Number 5 carbon would be above it. D means this five-carbon sugar has the same stereochemical configuration at the Number 4 and 5 carbons as the Number 2 and 3 Carbons of D-glyceraldehyde. "3',5' linkages" means that two deoxyribose sugars are connected to a phosphate in the middle by the Number 3 carbon on one sugar and by the Number 5 carbon on the other sugar. Characteristic 3: the two strands in DNA are anti-parallel. "Both chains follow right- handed helices, but owing to the dyad the sequences of the atoms in the two chains run in opposite directions." Dyad means the pairing of opposite bases. This is analogous to two teams shaking hands in two passing lines after a championship game. The two teams are in two lines moving in opposite directions. This allows people to shake each other's right hands. If the two lines were parallel instead of anti-parallel, would they be able to shake hands easily? "Right-handed helices" refers to the orientation of the helices. Compare to the threads on a screw. Standard threads are "right-handed." The alternative of course is "left-handed." I'd suggest using a nut and bolt to show how the handedness works. Occasionally you can even get "reverse-threaded" or "left-handed" hardware, but it's rare. "Right-handed" means a helix or screw that will move away from you if you turn a screwdriver clockwise. Some Calculations. "We have assumed an angle of 36 degrees between adjacent residues in the same chain, so that the structure repeats after 10 residues on each chain, that is, after 34 A. The distance of a phosphorus atom from the fibre axis is 10 A." Compare this to a circular staircase. In this analogy, you start out on stair 1; you look directly above you, and see a stair. To get to the stair directly above stair 1, you have to go up 10 stairsteps. "As the phosphates are on the outside, cations have easy access to them." The phosphates are negatively charged, and attract cations (net positive charge). The phosphates, being charged, are also hydrophilic. Characteristic 4: DNA is complementary. "The novel feature of the structure is the manner in which the two chains are held together by the purine and pyrimidine bases.... if only specific pairs of bases can be formed, it follows that if the sequence of bases on one chain is given, then the sequence on the other chain is automatically determined." Hydrogen bonding between the keto forms of A and T and between G and C explains complementarity and Chargaff's rules. To know one strand is to know the opposite strand. Note that this base-pairing model makes sense only if one assumes the bases are in the "keto" form. The "enol" form does not provide an opportunity for hydrogen bonding. "Keto" and "enol" are two possible tautomeric forms of a carbon in the bases. A carbon in the "keto" form is a carbon with a double bond to oxygen (as in "ketone"), and two single bonds to adjacent members of the ring. A carbon in the "enol" form has a single bond to an alcohol group (-OH) and a double-bond to a nitrogen and a single bond to an adjacent carbon. Hydrogen bonding is a key attribute of the Watson-Crick model. The opportunity for hydrogen bonding depends on whether the bases are in the "keto" or the "enol" form. Watson and Crick found that the AT and GC pairing make sense only if the bases are in the "keto" tautomeric form. The keto/enol dilemma is particularly well illustrated in Chapter 26 of Watson's "The Double Helix." (see book citation below) ic semper dyslexia: in the web version, the following misspellings occur: "the ration (sic) of guanine to cytosine, are always bery (sic) close to unity for deoxyribose nucleic acid." The misspellings seem to be a function of the website and not a verbatim copy of the original. Double-stranded RNA? What difference can an oxygen make? The authors write that "It is probably impossible to build this structure with a ribose sugar in place of the deoxyribose..." This implies doubt about the possibility of dsRNA. Yet now we know that dsRNA does exist routinely in nature, especially in some viruses of plants and fungi. Question: Is dsRNA comparable structure to dsDNA? It is known to be double-stranded, antiparallel and complementary. Is it also a double-helix? The Great Understatement: "It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material." The novel feature of the model--namely, complementarity---means that knowing one strand tells you what the opposite strand should be. This is a remarkable feature for a template. It also means that pulling the double-stranded DNA apart yields two single strands of DNA. And if you fill in the missing strand of each, merely following the Watson-Crick binding rules (A to T, T to A, G to C, C to G), you can get two copies of the original double-stranded DNA. Imagining this idea and generating ways to test it are two different challenges. It took another 5 years for Miselson and Stahl to develop the experiments that tested whether DNA was copied by "semi-conservative" or "conservative" replication. Their results, published in 1958, supported the semi-conservative mechanism. Taken from: http://www.accessexcellence.org/RC/AB/BC/casestudy2.php