* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Fatty acid metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Biosynthesis wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Genomic imprinting wikipedia , lookup
In vitro fertilisation wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Cryobiology wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
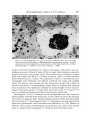
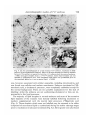
/ . Embryo/, exp. Morph. Vol. 33, 3, pp. 725-730, 1975 Printed in Great Britain 725 Autoradiographic studies of tn/tn mouse embryos By MARY NADIJCKA 1 AND NINA HILLMAN 1 From the Department of Biology, Temple University, Philadelphia SUMMARY High-resolution autoradiographic studies were used to determine whether t12/t12 and Z"'32//"32 mouse embryos synthesize the excessive lipid which distinguishes these embryos prior to their death. The studies show that the tn homozygotes synthesize neutral lipid which is stored in intracellular lipid droplets. Cholesterol and phospholipid precursors are not incorporated into these droplets. INTRODUCTION Three mutant recessive alleles (t12, tw32, t6), when homozygous, display similar phenotypic characteristics (Hillman, Hillman & Wileman, 1970; Hillman & Hillman, 1975; Nadijcka & Hillman, 1975). One of these characteristics is the presence of excessive cytoplasmic lipid prior to the lethal periods of the homozygous mutant embryos. Both t12/t12 and twS2/tu'32 embryos also exhibit nonphysiological levels of ATP metabolism (Ginsberg & Hillman, 1975). Since it has been reported that increased ATP synthetic rates can increase lipogenesis by shunting acetyl CoA into fatty acid synthesis (Atkinson, 1965; Newsholme & Start, 1973), it has been suggested that the aberrant ATP synthesis could result in the characteristic excessive lipid. The present high-resolution autoradiographic study was undertaken to determine first, if the excessive lipid was synthesized by the tnjtn embryos, and second, the type of lipid deposited. MATERIALS AND METHODS Standard crosses were used to obtain T+lt12 (Smith, 1956) and T+/tw32 (Bennett & Dunn, 1964; Hillman & Hillman, 1975) mice. Eight-week-old T+ltn (t12 or tw32) females were superovulated (Edwards & Gates, 1959) and mated to heterozygous males of the same genotype (Hillman et al. 1970; Hillman & Hillman, 1975). Pregnant females were sacrificed on gestation day 1 (day 0 = day of plug) and the 2-cell embryos were flushed from the excised oviducts with Brinster's medium and placed into culture (Brinster, 1963). Each litter contained more than the expected Mendelian ratio of homozygous 1 Authors'1 address: Department of Biology, Temple University, Philadelphia, Pennsylvania 19122, U.S.A. 726 M. N A D I J C K A AND N. H1LLMAN Fig. 1. An autoradiograph of a portion of a late morula t12jt12 embryo labelled from the 8-cell to late morula stage with [14C]pyruvate. Note the presence of tracks over the lipid droplets (L), myelin bodies (MB) and degradation bodies (DB). No tracks are associated with mitochondria (M). Similar labelling patterns are found in twS2/tu-32 a n d c o n t r o i morula embryos, x 18000. mutants since T+/tn males transmit the t12 and ?u32 alleles in frequencies higher than normal (Smith, 1956; Bennett & Dunn, 1964). Individual litters developed in the standard medium until they reached the early 8-cell stage when they were separately placed into radioactive medium. Litters containing tw3*ltw*2 embryos remained in the radioactive medium until they had reached the early morula stage of development (12 h of incubation) and litters containing t12 homozygotes were kept in radioactive medium until the late morula stage (24 h of incubation). The radioactive compounds used were [14C]pyruvate (Amersham/Searle, 12-4mCi/mM), [3H]ethanolamine(Amersham/ Searle, 320 mCi/mM), [3H]choline chloride (New England Nuclear, 1 Ci/mM), [3H]mevalonic acid (Amersham/Searle, 82 mCi/mM) and [3H]palmitic acid (Schwarz/Mann, 27 Ci/mM). Prior to use, the 3H-labelled precursors were diluted with Brinster's medium to a concentration of 5 /*Ci/ml medium and [14C]pyruvate was diluted to 2/*Ci/ml medium. After removal from culture, the embryos were washed in non-radioactive Autoradiographic studies o/t n /t n embryos 727 m Fig. 2. An autoradiograph of a tu'32jtwSZ embryo incubated from the 8-cell stage until the early morula stage in [3H]ethanolamine-supplemented medium. Label is scattered over the cytoplasm but is rarely found over the lipid droplets (L) of either the homozygous tn embryos or the control embryos, x 6500. medium and fixed immediately for electron microscopy. The fixation and embedding protocols of Stein & Stein (1971) for lipid high-resolution autoradiography were used in the present study. Thin sections were collected on copper grids and coated with Ilford L-4 nuclear emulsion. After a suitable exposure period (3H-precursors, 2 weeks; 14C-compound, 3 weeks to 3 months) the autoradiographs were developed with Dektol, acid-fixed and rinsed with distilled water. The sections were stained with lead citrate and viewed with a Zeiss 9 A electron microscope. Correspondingly staged litters of random-bred embryos were incubated in the radioactive medium for similar lengths of time and processed in the same manner. At least three litters from both T+/t12 and T+jtwZ2 inter se matings and three control litters were used for each study. Homozygous t12 and twZ2 embryos can be distinguished from their respective phenotypically wild-type litter-mates prior to their lethal periods by the presence of excessive cytoplasmic lipid. Nuclear fibrillo-granular bodies and binucleated cells are found in t12 homozygotes while tw32 homozygotes are characterized by abnormal mitochondria and binucleated cells (Hillman et ah 1970; Hillman & Hillman, 1975). These morphological characteristics were used, in the present study, to separate the homozygous tnjtn embryos from their litter-mates. 728 M. N A D I J C K A AND N. HILLMAN Fig. 3. A portion of a t12/t12 embryo incubated in [3H]choline chloride-supplemented medium from the 8-cell stage until the late morula stage. The pattern of labelling is the same as that found in tw32/tw32 embryos (Fig. 2). Silver grains are seldom found over the lipid droplets but are scattered over other cellular organelles and membranes, x 40000. RESULTS AND DISCUSSION Incubation in medium supplemented with [14C]pyruvate, which can serve as the sole energy source for the in vitro development of mouse embryos during cleavage (Brinster, 1965), was used to determine if the excessive lipid was a result of synthesis by the mutant embryo. Autoradiographs of embryos grown in [14C]pyruvate showed that most of the lipid was labelled in t12/t12 and tw*2ltw*2 embryos, in their respective phenotypically wild-type litter-mates and in correspondingly staged control embryos. This indicates that all of the embryos are synthesizing lipid during the later cleavage stages. The tn homozygotes contained more lipid than the control embryos and most of this excessive lipid was labelled. Label was also found scattered over all other cellular organelles, with the exception of mitochondria, as well as over the cytoplasm and nucleoplasm of the embryos. Fig. 1 shows a typical labelling pattern. Neither the excessive lipid in tn homozygotes nor the lipid droplets in control embryos were labelled after incubation in medium containing the phospholipid precursors, [3H]ethanolamine (Fig. 2) and [3H]choline chloride (Fig. 3). Label Autoradiographic studies o/t n /t n embryos 729 Fig. 4. (a) An autoradiograph of a late morula f12/'12 embryo, incubated in [3H]palmitic acid-supplemented medium. Most of the lipid droplets are heavily labelled (arrows). This pattern is typical for both the tn/tn embryos and the control embryos, x 3600. (6) Lipid droplets from a tu32/tuS2 embryo. Some lipid droplets are not labelled by [3H]palmitic acid. This unlabelled lipid either was synthesized prior to the treatment period or is not a neutral lipid. x 14000. was, however, associated with cellular organelles, including mitochondria, and was found over cellular and nuclear membranes. Embryos incubated in [3H]mevalonic acid, a cholesterol precursor, were completely unlabelled except for the normal background. There are two possible explanations for this lack of labelling: either the embryos are not synthesizing cholesterol or they are impermeable to this lipid precursor. The majority of lipid droplets in normal embryos and most of the excessive lipid deposits of the mutants were densely labelled following incubation in medium supplemented with the neutral lipid precursor, [3H]palmitic acid (Fig. 4). Those droplets which were not labelled may be assumed to be either composed of non-neutral lipid or synthesized during the earlier cleavage stages prior to incubation in radioactive medium (Fig. Ad). Silver grains were also found 730 M. NADIJCKA AND N. HILLMAN randomly scattered over the cytoplasm, nucleoplasm, membranes and cellular organelles. From the present series of experiments, it is impossible to determine whether the excessive lipid deposits, characterizing the homozygous tn embryos, result from excessive lipogenesis or from a lack of utilization of the lipid which is being synthesized at normal rates. To distinguish between these two possibilities embryos must be processed for autoradiography after a suitable chase period following their incubation in radioactive medium. We have found, however, that it is necessary to incubate the embryos in radioactive lipid precursors for extensive periods of time (from early 8-cell until early or late morula) for these embryos to be sufficiently labelled for autoradiographic analysis. In addition, mutant embryos must be incubated with radioactive label during the later cleavage stages since it is during these developmental periods that tnjtn embryos exhibit the greatest lipid deposition (Hillman et al. 1970; Hillman & Hillman, 1975). Since most of the tn/tn embryos die during the early (tw32) and late (t12) morula stages, it is not possible to use a chase period, and consequently it is not possible to determine the cause of their excessive lipid deposition. Nevertheless, the studies do show that both t12/t12 and tw32/tw32 embryos are synthesizing the excessive lipid and that this lipid is labelled only by the neutral lipid precursor. The research was supported by U.S. Public Health Research Grant HD-00827. The authors would like to acknowledge the technical assistance of Marie Morris and Geraldine Wileman. REFERENCES ATKINSON, D. E. (1965). Biological feedback control at the molecular level. Science, N.Y. 150, 851-857. BENNETT, D. & DUNN, L. C. (1964). Repeated occurrences in the mouse of lethal alleles of the same complementation group. Genetics 49, 949-958. BRINSTER, R. L. (1963). A method for in vitro cultivation of mouse ova from two-cell to blastocyst. Expl Cell Res. 32, 205-208. BRINSTER, R. L. (1965). Studies on the development of mouse embryos in vitro. II. The effect of energy source. /. exp. Zool. 158, 59-68. EDWARDS, R. G. & GATES, A. H. (1959). Timing of the stages of the maturation divisions, ovulation, fertilization and the first cleavage of eggs of adult mice treated with gonadotrophins. /. Endocr. 18, 292-304. n n GINSBERG, L. & HILLMAN, N. (1975). ATP metabolism in t /t mouse embryos. /. Embryol. exp. Morph. 33, 715-723. m 32 HILLMAN, N. & HILLMAN, R. (1975). Ultrastructural studies of t' lt"' mouse embryos. /. Embryol. exp. Morph. 33, 685-695. HILLMAN, N., HILLMAN, R. & WILEMAN, G. (1970). Ultrastructural studies of cleavage stage t12/t12 mouse embryos. Am. J. Anat. 128, 311-340. 6 G NADIJCKA, M. & HILLMAN, N. (1975). Studies of t lt mouse embryos. /. Embryol. exp. Morph. 33, 697-713. NEWSHOLME, E. A. & START, C. (1973). Regulation in Metabolism. New York: John Wiley & Sons. SMITH, L. J. (1956). A morphological and histochemical investigation of a preimplantation lethal (f12) in the house mouse. /. exp. Zool. 132, 51-83. STEIN, O. & STEIN, Y. (1971). Light and electron microscopic radioautography of lipids: techniques and biological applications. In Advances in Lipid Research, vol. 9 (ed. R. Paoletti & D. Kritchevsky), pp. 1-72. New York and London: Academic Press. (Received 29 July 1974)