* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Novel physiological and metabolic insights into the beneficial
Pharmacometabolomics wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Citric acid cycle wikipedia , lookup
Blood sugar level wikipedia , lookup
Electron transport chain wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Butyric acid wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
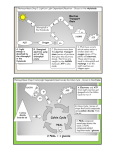
University of Groningen Novel physiological and metabolic insights into the beneficial gut microbe Faecalibacterium prausnitzii Khan, Muhammad Tanweer IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2013 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Khan, M. T. (2013). Novel physiological and metabolic insights into the beneficial gut microbe Faecalibacterium prausnitzii: from carbohydrates to current Groningen: s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 18-06-2017 Chapter 6 Metabolic switch upon transition from an electrogenic phase to fermentation in the human gut bacterium Faecalibacterium prausnitzii M. Tanweer Khan1, Wesley R. Browne2, Jan Maarten van Dijl1, Hermie J.M. Harmsen1 1 Department of Medical Microbiology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands 2 Stratingh Institute for Chemistry, Faculty of Mathematics and Natural Sciences, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherland Submitted to Energy Environ. Sci Chapter 6 Abstract Faecalibacterium prausnitzii is one of the most abundant commensal microbes in the gut of healthy humans, and this bacterium is well-known for its beneficial effects on gut inflammation. Previous studies revealed that F. prausnitzii can efficiently exploit flavins as redox mediator to shuttle electrons to external electron acceptors. The present studies were aimed at elucidating the mechanism and impact of riboflavin-mediated extracellular electron transfer (EET) on the growth and metabolism of F. prausnitzii in microbial fuel cells (MFCs). Using chronoamperometry, bacterial cells were cultured at different anodic potentials and their metabolic profiles were assessed. Furthermore, resting cells were used to investigate possible connections between metabolic flux and current-producing pathways. The results show that at highly oxidizing potentials, F. prausnitzii exploits riboflavin as electron shuttle to dissipate the reducing equivalents that is generated by glycolysis and a presumed pyruvate:ferredoxin oxidoreductase system. The growth profile in the MFCs further reveals an initial current-producing electrogenic phase that is followed by a fermentation phase. Notably, carbon flux is regulated during the electrogenic phase with a part of the glycolytic flux being utilized for the biosynthesis of an extracellular polymeric substance (EPS). Altogether, the present study shows that the metabolic flux in F. prausnitzii is modulated towards EPS production in response to oxidizing environments, and that the internal redox balance is maintained via EET. Based on these observations, we hypothesize that F. prausnitzii uses EPS and EET for colonization of the gut mucosa and protection against the oxidative conditions that can be encountered in this niche. 132 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Introduction The ‘gut microbiota’ is of fundamental importance to human health and well-being due to its critical roles in the recovery of energy from undigested food, immune regulation and colonization resistance against pathogens. Faecalibacterium prausnitzii is one of the most abundant gut microbes, representing about 20% of the total faecal microbiota in healthy individuals 1. Recently, metabolic and physiological studies have revealed major contributions of F. prausnitzii to gut health, relating to the production of butyrate and potentially strong antiinflammatory compounds 2-4. The major product of glucose fermentation by F. prausnitzii is butyrate, but formate and lactate are also produced at lower levels 5. In this case, the glycolytic pathway catabolizes glucose to pyruvate, generating ATP and the reduced electron carrier NADH (equation 1). The subsequent conversion of pyruvate to acetyl-CoA is either catalysed by pyruvate:ferredoxin oxidoreductases (PFOR; equation 2) that generate reduced ferredoxin (Fdred) and CO2 as additional products, or by pyruvate formate lyase that generates formate as an additional product (equation 3). AcetylCoA is then converted into butyrate through a series of enzymatic reactions involving the uptake of extracellular acetate (equation 4) 6,7 . Thus, glucose consumption and butyrate production proceed in a 1:1 stoichiometric ratio if no lactate or acetate are produced through the respective activities of lactate dehydrogenase (LDH) and acetate kinase (ACK) 8. A tentative model for the metabolic fluxes in F. prausnitzii is presented in Figure 1. This model takes into account the established biochemistry of the central carbon metabolism in bacteria belonging to the Clostridium group to which F. prausnitzii belongs 6,9. 133 | P a g e Chapter 6 134 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Figure 1. Proposed Metabolic flux network of F. prausnitzii based on Patni & Alexander 19719 and Seedorf et al., 20086. The EPS biosynthetic pathway was included in this network based on the observations presented in this study and blast searches, which revealed the presence of genes for EPS synthesis (data not shown). Etf, electron transport flavoproteins; EPS, extracellular polymeric substance; F1F0, ATP synthase; F-6-P, fructose 6-phosphate; G-1-P, Glucose 1-phosphate; G-6-P, Glucose 6-phosphate; IDP-G, Isoprenoid diphosphate glucose; PEP, Phosphoenol pyruvate; RnF complex, electron transferring ferredoxin:NAD oxidoreductase; a UDP-G, uridine diphosphate glucose; , indicates hypothetical redox enzymes that mediate EET and proton translocation across the membrane. 135 | P a g e Chapter 6 Theoretically, butyrate production disposes of around 83% of the total electrons derived from glucose catabolism, while generating NAD+ and Fdred (equation 5). The proton-translocating NAD+:ferredoxin oxidoreductase complex (NAD+: FOR or Rnf complex) in the membrane is then exploited to regenerate oxidized ferrodoxin (Fdox) from Fdred at the expense of NAD+ (Fig. 1) 7,10 . This NAD+ dissipation might influence the glycolytic flux, since NAD+ is the crucial oxidant in this pathway 11. Alternatively, Fdox could be regenerated from Fdred by the activities of a hydrogenase, which would result in hydrogen production 10,12,13. However, the generation of hydrogen gas was thus far not detected in in vitro fermentations of F. prausnitzii and, therefore, Fdox recycling via this route remains ambiguous 5. Importantly, electron disproportionate conditions where excess of reduced products, such as Fdred or NADH, might accrue must be firmly regulated. To cope with such conditions, microbes have evolved subtle mechanisms to couple their endogenous oxidative metabolism (i.e. the regeneration of NAD+) to external reductive reactions, such as the reduction of metal complexes, via a process called ‘extracellular electron transfer’ (EET) 14. Since electrons cannot traverse the lipid bilayer spontaneously, EET relies on distinct components, such as electron shuttles/mediators, membrane bound cytochromes, or nanowires . Biological 15-17 electrochemical systems, such as microbial fuel cells (MFCs), in which electrodes are used as the extracellular electron acceptor, represent highly effective tools to study EET. Interestingly, previous investigations with MFC systems have revealed changes in microbial metabolic profiles when different anodic potentials were applied 18,19. Electron mediators can be employed in MFCs to facilitate the electron transfer between microbial cells and the electrode 20. Accordingly, we have recently shown that riboflavin can be used to study EET by F. prausnitzii in MFC systems 21. This was important, because our earlier studies had revealed that F. prausnitzii employs riboflavin-mediated EET to consume oxygen in moderately oxygenated environments, such as the gut mucosa. By doing so, this highly oxygen-sensitive 136 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii bacterium creates a niche in which it can both survive and thrive 22. In the present studies, we performed combined EET and metabolic investigations on F. prausnitzii cells growing in MFCs to obtain novel insights in the mechanisms that determine fermentative end-product changes. Essentially, F. prausnitzii was used as a biocatalyst with glucose as electron donor and riboflavin as electron mediator to couple the bacterium's oxidative metabolism to the MFC electrode. Electronic parameters and fermentation profiles were then compared under electrogenic and non-electrogenic conditions. Interestingly, production of an extracellular polymeric substance (EPS), typically composed of polysaccharides, was observed under oxidizing conditions. Since it has been proposed previously that EPS production by commensal gut microbes may have a beneficial role in the maintenance of guthealth 23 , and since EPS production and central carbon metabolism are directly connected 24 , we focused particular attention on the modulation of the metabolic flux towards EPS production by F. prausnitzii. Altogether, our findings show that the internal redox balance of F. prausnitzii is maintained via EET and that the metabolic flux is directed towards EPS production in response to oxidizing environments. 137 | P a g e Chapter 6 Materials and Methods Bacterial strains and culturing conditions F. prausnitzii strain A2-165 (DSM 17677) was maintained at 37o C on yeast extract, casitone, fatty acid and glucose (YCFAG) agar in an anaerobic tent. For the microbial fuel cell experiments, the bacterial cells were grown anaerobically in 5 ml of YCFAG broth to an optical density at 600 nm (OD600) of ~0.8 5. Cells were harvested by centrifugation, washed with fresh YCFAG medium and injected (2.5% v/v inoculum) into the anode chamber using a gas tight syringe. Modified YCFAG medium (mYCAG) was used as anolyte. The mYCAG medium was similar to the YCFAG medium, but all short-chain fatty acids (SCFAs) except acetate were omitted, cysteine was reduced to 3 mM and 200 µM riboflavin was included as a redox mediator. For experiments with resting cells, cell cultures were prepared as described previously 21. Microbial fuel cell and electrical parameters A custom-made two-chambered, MFC was fabricated from borosilicate glass bottles with 250 ml and 100 ml working volumes for the anode and cathode chambers, respectively (Supplementary Fig. 1). The two compartments were separated by a CMI-7000S cation exchange membrane (Membranes International, Inc.), using a 20 cm diameter septum. A graphite slab (dimensions of 8.5 x 2.5 x 0.5 cm3) was used as anode, a 0.5 mm diameter platinum wire as auxiliary electrode and an Ag/AgCl wire was used as reference electrode (in the anode chamber). The anode was connected to the external circuit via copper wires and the bare ends were sealed with non-conducting epoxy resin. The self-resistance between anode and copper wire was less than 2 Ω. The mYCAG medium, which contains 200 µM riboflavin as a redox mediator, was used as anolyte while 100 mM potassium phosphate buffer was used as catholyte. A potentiostat (model # 600C, CH Instruments, Austin, TX, USA) was used to apply external anodic potentials. The selected potentials were +0.62 V, +0.35 V, +0.15 V and -0.35 V 138 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii versus Ag/AgCl, corresponding to +0.82 V,+0.55 V, +0.35 V and -0.15 V versus the standard hydrogen electrode (SHE), respectively. The potentiostat was operated in bulk electrolysis mode and the catalytic current was measured at intervals of 30 s for 24 h. After 24 h of growth in the MFC, cyclic voltammetry was conducted using the afore-mentioned potentiostat without exchange of the spent broth. The anode was used as working electrode, the Ag/AgCl wire as reference electrode, and the platinum wire cathode as auxiliary electrode. The selected scanning potential range was -0.5 V to -0.8 V versus Ag/AgCl and a scan rate of 50 mV s-1 was employed. MFC experiments with resting cells were performed as previously described 21. However, these experiments were conducted at an applied anodic potential of +0.15 V versus Ag/AgCl. Furthermore, nonelectrogenic controls (i.e. without electrode) were also executed. Live/dead staining for detection of biofilm formation on the working electrode was performed as previously described25. For energizing the resting cells, glucose, glucose plus acetate, and pyruvate were used. The 200 µM riboflavin was added as a redox mediator. The resting cells were incubated for 1.5 h. Analysis of short-chain fatty acids and carbohydrates A high-performance liquid chromatography (HPLC) Ion-Chromatography system (MetrohmAG, Herisau, Switzerland) equipped with a conductivity detector was used for measuring SCFA concentrations including lactate, formate, butyrate and acetate. A 6.1005.200 Metrosep Organic Acids column (Metrohm AG) with 9 µ m particle size and dimensions of 7.8 mm x 250 mm was used for SCFA separation. The eluent was 0.5 mM sulfuric acid and acetone (98:2 v/v), and the flow rate was 0.7 ml/min. Glucose consumption was estimated using the 3,5-dinitrosalicylic acid (DNSA) method 26 . Secreted EPS was extracted from the growth medium as described previously 27 and total carbohydrate content was determined by the phenol-sulphuric acid method 28. 139 | P a g e Chapter 6 Results and Discussion Metabolite profiles of F. prausnitzii cells grown at different redox potentials indicate a metabolic switch upon the transition from an electrogenic phase to fermentation To investigate the influence of environmental redox states on the metabolism of F. prausnitizii, cells were grown in a MFC at four different anodic potentials. In addition, a control experiment was performed in a MFC without electrode. When grown on mYCAG medium in the absence of an electrode, F. prausnitzii fermented glucose into lactate, formate and butyrate as previously described for the batch fermentation of this bacterium in YCFAG medium 8. Figure 2a summarizes the observed bacterial growth (right panel) as well as the profiles for glucose consumption and the production of butyrate, formate and acetate (left panel). These data show that the production of butyrate and formate was proportional to glucose consumption. It was furthermore observed that the formate production rate (1.45 mM. h-1) during the exponential phase was around 1.6-fold higher than the butyrate production rate (0.95 mM. h-1). Importantly, it was previously established that bacterial cells harvested from exponentially growing or stationary phase cultures exhibit no significant differences in the activities of enzymes required for the final steps in butyrate formation 8. Therefore, the higher rate of formate production is indicative of a high glycolytic flux at the pyruvate node (Fig. 1) that will cause an accumulation of glycolytic intermediates and reduced electron carriers, such as NADH 29. The reoxidation of NADH through butyrate production requires Fdox 7. In general, bacteria of the Clostridium group produce high Fd levels, and the reduced Fd forms are commonly recycled via hydrogenases or hydrogenase-bifurcating enzymes, generating Fdox and NAD+ simultaneously 30. 140 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Figure 2. Metabolic profiles under electrogenic and non-electrogenic growth conditions in a Microbial Fuel Cell system. a, growth in the absence of an electrode; b, growth at -0.35 V; c, growth at +0.15 V; d, growth at +0.35 V; e, growth at +0.62 V. All applied voltages were measured against an Ag/AgCl reference electrode. Left panels show extracellular concentrations of glucose and short-chain fatty acids (in mM) and catalytic current production (in mA). Right panels show growth measured by OD600 readings and the production of EPS (in mg/ml). 141 | P a g e Chapter 6 However, faecalibacteria do not seem to exploit such hydrogenases for the regeneration of Fdox, which implies that the NAD+:FOR route is used for this purpose (Fig. 1, depicted as Rnf complex). This recycling of Fdox via the NAD+:FOR route can result in high NADH/NAD+ ratios during the anaerobic growth of F. prausnitzii 10,30 . During cultivation of F. prausnitzii in the MFC without an electrode, acetate consumption in the early exponential growth phase was followed by acetate production in the late exponential and early stationary growth phases. The initial acetate consumption has been linked to the activity of a butyryl-CoA:acetate-CoA transferase (Fig. 1)31,32. In contrast, the subsequent acetate production is catalyzed by ACK, which generates additional ATP via substrate level phosphorylation 6,8 . Previous studies have shown that ACK was activated or produced during the stationary phase, which may relate to an increased flux at the acetyl-CoA node in central carbon metabolism 8. Furthermore, during, the early stationary phase, low levels of lactate were detected in the growth medium, indicating a build-up of intracellular glycolytic intermediates and reduced electron carriers (Figs. 1 and 2a) 30. Intriguingly, the bacteria secreted substantial amounts of EPS into their growth medium during the lag- and exponential growth phases (Fig 2a, right panel). This EPS was subsequently degraded, but it reappeared during the stationary phase, which may relate to hindrance in the carbon and electron flow as discussed in the following sections. The overall carbon and electron recoveries under these conditions were ~90% after 16 h of growth (Supplementary Table 1a). Next, the effects of different applied electrochemical potentials on the growth and metabolic profiles of F. prausnitzii were investigated. To this end, the bacteria were grown in the MFC at applied potentials of -0.35 V, 0.15 V, 0.35 V or 0.62 V versus Ag/AgCl. When an anodic potential of -0.35 V was applied, no catalytic current was observed. Instead, a negative current was observed (Fig. 2b, left panel). This was due to the reduction riboflavin as determined by cyclic voltammetry (-0.2 V versus Ag/AgCl; Fig. 3c). Under these conditions, the growth rate of F. 142 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii prausnitzii was slower than in the MFC without an electrode (Fig. 2a and b, compare right side panels), but the profiles for glucose, formate, butyrate, acetate and lactate remained typical for fermentative growth (Fig. 2b, left panel; Supplementary Table 1b). Also, EPS was produced during the exponential growth phase, consumed during the early stationary phase and produced again in the later stationary phase (Fig. 2b, right panel). Figure 3. Electrode pacification by biofilm formation. a, Photograph of a graphite working electrode pacified via biofilm formation. b, Live/dead staining of a F. prausnitzii biofilm on a working electrode; the green and orange fluorescence indicates live and dead cells, respectively. c, Cyclic voltammogram of a working electrode before (i) and after (ii) chronoamperometry. 143 | P a g e Chapter 6 When a potential of +0.15 V (versus Ag/AgCl) was applied, a catalytic current was observed (Fig. 2c, left panel) that represents the riboflavin-mediated flow of electrons from the bacterial cells to the anode as previously described 21. After ~15 h of growth, a maximum current of 17.6 mA was observed and, subsequently, the current dropped to lower levels. During the 'electrogenic' phase, before the maximum current was reached, the current was correlated to glucose consumption (Fig. 2c). It is presently not clear why the catalytic current declined after 15 h of growth, but this could be due acidification of the growth medium, or pacification of the electrode via the formation of a biofilm that interrupts electron transfer between bacterial cells and the electrode 33, or to a combination of these effects. The possible pacification of the working electrode was probed using live/dead staining, which revealed the presence of a biofilm (~15 µm thick) that entraps the projected surface area of electrode (Fig. 3, a and b). Analysis of the anode by cyclic voltammetry revealed a shift of ~60 mV in the potential difference between the oxidation and reduction peaks of riboflavin, indicating that the phenomenon of flavin adsorption on the anode may have occurred 34. Additionally, the pH of the medium was not controlled during MFC operation. Thus, the acidification of the growth medium may have contributed to the shift in the redox peaks of riboflavin possibly due to the floating potential. Interestingly, in previous studies it was shown that other electrogenic bacteria, such as Geobacter spp. or Shewenella spp., form anodic biofilms that positively correlate to power generation through direct electron transfer to the anode or through self-secreted redox mediators 35,15. On the other hand, Geobacter spp. and Shewenella spp. lack the ability to use glucose as an electron donor. Thus, the behaviour of F. prausnitzii in MFCs is very different from that of Geobacter spp. and Shewenella spp. as no direct electron transfer to the anode was observed in our present experiments. Moreover our previous studies have shown that F. prausnitzii requires glucose and externally added riboflavin for the generation of a catalytic current 21. 144 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Notably, at a potential of +0.15 V, glucose consumption, EPS production and current generation by F. prausnitzii started long before SFA production was detectable (Fig. 2c, left panel). Moreover, the overall carbon/electron recoveries remained low (~50%) as compared to the control experiment in a MFC without electrode, where they amounted ~90% (Supplementary Table 1a, b and c). Together, these observations reveal an important switch in the metabolism of F. prausnitzii between the predominantly electrogenic initial growth phases and the subsequent fermentative growth phases. The increasing current generated by EET before the start of the fermentative phase indicates a dissipation of the reducing power generated by glycolysis due to NADH oxidation 10,19 . This would explain the retardation of SCFA production since both butyrate and lactate production require NADH as reducing equivalent (Fig. 1) 6. Furthermore, since no formate was detectable before the onset of the fermentative growth phase, it seems that glycolytic intermediates were shuttled away from the pyruvate node of central carbon metabolism. This is in line with the previous observation that the pyruvate formate lyase enzyme complex catalyses formate production independent of reducing energy consumption 6,22 . Together, these findings suggest that, at a potential of +0.15 V, glycolytic intermediates are directed into the EPS biosynthetic pathway during the early electrogenic growth phase, as depicted in Figure 1. This view is consistent with studies on EPS formation by Staphylococcus epidermidis, where it was shown that inhibition of the TCA cycle resulted in a modulation of the redox state of the cells, possibly due to higher NADH/NAD+ ratios, and a concomitant accumulation of glycolytic intermediates that facilitated the biosynthesis of EPS 36. Furthermore, the data in Figure 2c reveal that during the fermentative phase, starting after ~15 h of growth, formate and butyrate production occurred at similar rates without the simultaneous production of lactate. These SCFA productions rates are thus very different from those that were observed for the control conditions (no electrode present or -0.35 V), where the formate production rate was higher than the butyrate production rate, and where 145 | P a g e Chapter 6 lactate was also produced (Fig. 2, compare panels a, b and c). This underscores the view that, during electrogenic growth, the accumulation of glycolytic intermediates is lower than under fermentative growth, and that reduced electron carriers are reoxidized with the help of EET while glycolytic intermediates are used for the biosynthesis of EPS 18,19,37. When potentials of +0.35 V or +0.62 V were applied, concomitant with the growth of F. prausnitzii a rapid increase in catalytic current was observed (Fig. 2, d and e). As noted for growth at +0.15 V, rapid glucose consumption was observed before significant levels of fatty acid production were detectable. The maximum catalytic currents observed at potentials of +0.35 V and +0.62 V were 12 mA (at ~9 h) and 9 mA (at ~6 h), respectively. Thus, the peak values of the currents obtained were lower than the peak value of 17.6 mA observed at a potential of +0.15 V (Fig. 2). These findings indicate that at relatively high potentials, rapid EET mediates an earlier peak in current generation and allows the faecalibacteria to grow at higher rates. Furthermore, at high potentials the cells acquired glucose relatively rapidly, allowing them to regulate their internal redox state by producing more reducing equivalents. In turn, this would allow a concomitantly increased generation of current, biomass and EPS, resulting in an earlier pacification of the electrode (Fig. 2, d and e) and preventing the MFC system from attaining higher catalytic currents. Most likely, the pacification of the electrode causes an increased intracellular level of glycolytic intermediates and reducing equivalents, which triggers a more rapid onset of fermentation (Fig 2, d and e; Supplementary Table 1, d and e). Indeed, the observed formate to butyrate production ratios at potentials of +0.35 V and +0.62 V were 1.3 and 1.8, respectively, which is close to the ratio of 1.6 observed in the control experiment where no electrode was present and the cells could grow exclusively fermentatively. Taken together, these observations indicate that a major metabolic switch occurs in F. prausnitzii upon the transition from the electrogenic phase to fermentation, which impacts directly on the formation of EPS. 146 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Resting cells of F. prausnitzii show differential metabolic fluxes under electrogenic and non-electrogenic conditions To obtain a better understanding of the current-generating pathway, resting cells of F. prausnitzii were used for MFC experiments. In these experiments, cells grown in YCFAG broth were harvested in the stationary growth phase and injected into a MFC system. Subsequently, 25 mM glucose, 25 mM glucose plus 50 mM acetate, or 50 mM pyruvate were added to energize the cells. As shown in Figure 4A, the steady state current generated in the presence of glucose was 1.6-fold higher than when the cells were incubated with glucose plus acetate (Fig. 4a). Under both of these current-producing conditions, so irrespective of the presence of acetate, the production of formate, acetate and butyrate was observed, whereas lactate production remained undetectable (Fig. 4, a, b and c). This indicates that NADH, which is needed as a reductant for lactate generation (Fig. 1), was dissipated via EET. Moreover, the cells avoided a possible blockage in carbon flux at the pyruvate node through the production of formate, acetate and butyrate. Interestingly, the lactate homofermentor Lactococcus lactis shows a similar metabolic shift towards mixed acid fermentation when grown under electrogenic conditions, which is possibly due to a high carbon flux at the pyruvate node 18 . When glucose plus acetate were added to the MFC, fatty acid production by F. prausnitzii was several folds higher than when only glucose was added (Fig 4, b and c). This indicates that a higher flux of carbon and reducing energy was directed towards SCFA production when acetate was present 8,38 , which would explain the lower steady-state current production by cells incubated with glucose and acetate (Fig. 4, a, b and c). Interestingly, when cells were incubated with glucose only under non-electrogenic conditions (i.e. no electrode was present in the MFC), the production of formate, acetate and butyrate was significantly reduced while lactate production was increased (Fig. 4, b and c). This indicates that the accumulation of reducing equivalents in the absence of EET hinders the glycolytic flux and that the cells started to produce lactate for NAD+ recycling 18,39. When pyruvate was added to the resting F. prausnitzii cells, an almost 3-fold lower 147 | P a g e Chapter 6 steady state current was observed than when glucose was added (Fig. 4a). This is in line with the fact that pyruvate generates four-fold less reducing equivalents than glucose (Fig. 1) 6. Furthermore, under electrogenic conditions, pyruvate was found to generate less lactate and more formate and acetate as compared to the non-electrogenic control conditions where no electrode was present (Fig. 4d). Importantly, under both electrogenic and non-electrogenic conditions, butyrate production was halted, even when 50 mM of acetate was added (data not shown). These observations indicate that the NADH, which is critical for butyrate production, was dissipated either via EET and lactate production under electrogenic conditions, or via lactate production under non-electrogenic conditions 10,12,13. Furthermore, the efficient lactate and acetate production prevents the blockage of central carbon metabolism at the pyruvate and acetyl-CoA nodes, thereby diverting the carbon flux away from butyrogenesis. Under electrogenic conditions, the lower level of lactate production due to the utilization of NADH for EET seems to enhance the carbon flux at the pyruvate node towards acetyl-CoA production, which would explain the enhanced production of formate and acetate (Fig. 4d). Together, these findings show that fluxes in the central carbon metabolism of F. prausnitzii are very different under electrogenic and nonelectrogenic conditions. 148 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Figure 4. Current production and metabolic profiles of resting F. praunitzii cells in the microbial fuel cell. Panel a. Cells were fed with either: i, glucose (25 mM); ii, glucose (25 mM) plus acetate (50 mM); or iii, pyruvate (50 mM), chronoamperometry was carried out at a potential of +0.15 V versus Ag/AgCl. Panels b, c & d. Metabolic profiles of resting cells using glucose, glucose/acetate or pyruvate as electron donors under electrogenic (dark bars) or fermentative conditions (light bars). Note that in the experiment where glucose plus acetate were added, the net acetate production was calculated by subtracting the base line value at t=0. 149 | P a g e Chapter 6 Energetics of riboflavin-coupled Extracellular Electron Transport To assess the possible advantage of EET for F. prausnitzii, the energetics of EET and ATP gain were calculated from the Gibb’s free energy of the electron transfer between NADH and riboflavin based upon the following considerations. During glucose fermentation, F. prausnitzii produced mainly formate and butyrate, as well as some lactate (Fig. 2). In contrast, SCFA production was retarded during the electrogenic phase, despite glucose consumption. Thus a major proportion of energy must have been derived from glycolysis, while EET mediated the recycling of NAD+ (Fig. 1). Consequently, during the metabolism of glucose to acetyl-CoA, eight electrons were respired to the anode of the MFC, thereby potentially generating ATP via the generation of a proton-motive force and an F-type ATP synthase 7,13 . Since the electrons generated during the conversion of glucose to acetyl-CoA are bound to electron carriers, such as NADH (Eo’ -0.32 V), a Gibbs free energy of ~85 kJ/mol can be estimated for the reduction of riboflavin (Eo’ 0.21 V). This free energy change can generate 1.5 mol of ATP per mole of glucose assuming an ATP synthesis cost of ~60 kJ/mol ATP. Thus, at a current of 17 mA, F. prausnitzii can synthesize ATP at a rate of ~33 nmol/s 10,13,15,34. A similar rate of ATP synthesis was estimated for Shewanella spp., which transfers electrons to the anode with the help of secreted riboflavin during the metabolic conversion of lactate to acetate 34. It thus seems that faecalibacteria can potentially gain 3.5 mol of ATP/mol of glucose through glycolysis combined with EET, without producing SCFAs. Implications of flavin-mediated electron shuttling The present studies provide novel insights into riboflavin-mediated extracellular respiration and the modulation of metabolic fluxes in F. prausnitzii. As shown in previous studies, this bacterium exploits an NAD+:FOR system to regenerate Fdox rather than hydrogenases. Consequently, the recycling of produced NADH to NAD+ via EET is an attractive option (Fig. 1) 7,13. However, faecalibacteria do not produce extracellular riboflavin and, therefore, they need riboflavin from external 150 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii sources as electron mediator 21,22 . This external riboflavin can then be used to reduce locally available electron acceptors, such as Fe3+ complexes, oxidized thiols or oxygen 34,21,22 . In MFCs, this phenomenon allows for catalytic current production, since the anode acts as a terminal electron acceptor. Interestingly, at relatively high positive anodic potentials, flavin-mediated respiration allows for rapid consumption of glucose, growth and pacification of the electrode, which causes a switch back towards the fermentative pathway. Additionally, during electrogenic growth the faecalibacteria produce EPS as a metabolic by-product, which possibly facilitates regulation of the glycolytic flux. Our present experiments with resting cells clearly show that the reducing energy for EET during the electrogenic phase was mainly derived from glycolysis and the subsequent conversion of pyruvate to acetyl-CoA (Figs. 1 and 4). Based on these findings, we propose the model depicted in Figure 5 to explain how faecalibacteria can undergo a metabolic switch from an electrogenic to a fermentative phase. A central element in this model is the view that riboflavin-mediated EET provides an efficient means for electron disposal and Fdox recycling. This is in line with the fact that F. praunitzii is a common inhabitant of the human gut, where considerable amounts of flavins derived from either diet or the local microbiota are readily available. Similarly, this explains why F. prausnitzii requires yeast extract as an obligatory component in its growth media , since the flavins contained in yeast 8,38 extract can be exploited as redox mediator for EET 40 . During the electrogenic growth, the cells produce no SCFAs indicating that the fermentative metabolism is shut down due to the dissipation of NADH for EET. This is energetically feasible, because our calculations imply that the Gibbs free energy available from riboflavin-coupled respiration can provide an extra gain of 1.5 mol of ATP per mol of glucose. Importantly, this will give faecalibacteria a competitive advantage over other gut bacteria (or other faecalibacteria) that grow purely fermentatively. In fact, this is exactly what we observed in our previous experiments where, compared to anaerobically growing faecalibacteria, the faecalibacteria in microaerobic 151 | P a g e Chapter 6 environments showed significantly enhanced growth, provided that EET to oxygen was possible due to the presence of riboflavin and oxidized thiols 21,22 . When EET in the MFC system is blocked due to pacification of the electrode by bacterial cells and EPS, F. prausnitzii switches to fermentative growth that results in the formation of SCFAs (Figure 5, right panel). Thus, our findings explain how F. prausnitzii deals with the external oxidative stress via EET and how it manipulates the central carbon flow by EPS production. It is tempting to translate these findings to the in vivo situation, where EET can be facilitated by riboflavin and thiols that are present in the gut lumen and oxygen diffuses from epithelial cells into the gut mucosa. This situation would facilitate electrogenic growth of F. prausnitzii, giving this bacterium a competitive advantage over other gut bacteria that are incapable of EET and creating a protective niche for host microbial interaction. Once EET would become impaired, for example due to EPS formation, the faecalibacteria would switch to fermentative growth thereby producing butyrate, which is the preferred energy source for colonocytes and has a potentially protective role against colitis and colorectal cancers. 152 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Figure 5. Proposed metabolic switch during the transition from electrogenic to fermentative growth as based on the present studies. See text for details. Acknowledgements: M.T.K. was supported by a grant from the Graduate School GUIDE of the University of Groningen. 153 | P a g e Chapter 6 References: 1. Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. 2. Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183-1189. 3. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an antiinflammatory commensal bacterium identified by gut microbiota analysis of crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731-16736. 4. Lopez-Siles M, Khan TM, Duncan SH, Harmsen HJ, Garcia-Gil LJ, Flint HJ. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl Environ Microbiol. 2012;78:420-428. 5. Duncan SH, Hold GL, Harmsen HJ, Stewart CS, Flint HJ. Growth requirements and fermentation products of fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol. 2002;52:2141-2146. 6. Seedorf H, Fricke WF, Veith B, et al. The genome of clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc Natl Acad Sci U S A. 2008;105:2128-2133. 7. Herrmann G, Jayamani E, Mai G, Buckel W. Energy conservation via electrontransferring flavoprotein in anaerobic bacteria. J Bacteriol. 2008;190:784-791. 8. Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A (CoA):Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68:51865190. 9. Patni NJ, Alexander JK. Utilization of glucose by clostridium thermocellum: Presence of glucokinase and other glycolytic enzymes in cell extracts. J Bacteriol. 1971;105:220-225. 10. Wang S, Huang H, Moll J, Thauer RK. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electronbifurcating enzyme complex in clostridium kluyveri. J Bacteriol. 2010;192:51155123. 154 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii 11. Bar-Even A, Flamholz A, Noor E, Milo R. Rethinking glycolysis: On the biochemical logic of metabolic pathways. Nat Chem Biol. 2012;8:509-517. 12. Li F, Hinderberger J, Seedorf H, Zhang J, Buckel W, Thauer RK. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from clostridium kluyveri. J Bacteriol. 2008;190:843-850. 13. Buckel W, Thauer RK. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation. Biochim Biophys Acta. 2013; 1827:94-113 14. Watanabe K, Manefield M, Lee M, Kouzuma A. Electron shuttles in biotechnology. Curr Opin Biotechnol. 2009;20:633-641. 15. von Canstein H, Ogawa J, Shimizu S, Lloyd JR. Secretion of flavins by shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol. 2008;74:615-623. 16. Clarke TA, Edwards MJ, Gates AJ, et al. Structure of a bacterial cell surface decaheme electron conduit. Proc Natl Acad Sci U S A. 2011;108:9384-9389. 17. Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:10981101. 18. Freguia S, Masuda M, Tsujimura S, Kano K. Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry. 2009;76:14-18. 19. Wang YF, Masuda M, Tsujimura S, Kano K. Electrochemical regulation of the end-product profile in propionibacterium freudenreichii ET-3 with an endogenous mediator. Biotechnol Bioeng. 2008;101:579-586. 20. Park DH, Zeikus JG. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl Environ Microbiol. 2000;66:1292-1297. 21. Khan MT, Browne WR, van Dijl JM, Harmsen HJ. How can Faecalibacterium prausnitzii employ riboflavin for extracellular electron transfer? Antioxid Redox Signal. 2012;17:1433-1440 155 | P a g e Chapter 6 22. Khan MT, Duncan SH, Stams AJ, van Dijl JM, Flint HJ, Harmsen HJ. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012;6:1578-1585. 23. Sims IM, Frese SA, Walter J, et al. Structure and functions of exopolysaccharide produced by gut commensal lactobacillus reuteri 100-23. ISME J. 2011;5:1115-1124. 24. Svensson M, Waak E, Svensson U, Radstrom P. Metabolically improved exopolysaccharide production by streptococcus thermophilus and its influence on the rheological properties of fermented milk. Appl Environ Microbiol. 2005;71:6398-6400. 25. Berney M, Hammes F, Bosshard F, Weilenmann HU, Egli T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl Environ Microbiol. 2007;73:3283-3290. 26. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 1959;31:426-428. 27. Annamaria Ricciardi, Eugenio Parente, Maria Aquino and Francesca Clementi. Use of desalting gel for the rapidseparation of simple sugars fromexopolysaccharides produced by lacticacid bacteria. Biotechnology Techniques. 1998;12:649-652. 28. Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350-356. 29. Roberts SB, Gowen CM, Brooks JP, Fong SS. Genome-scale metabolic analysis of clostridium thermocellum for bioethanol production. BMC Syst Biol. 2010;4:31. 30. Cai G, Jin B, Monis P, Saint C. A genetic and metabolic approach to redirection of biochemical pathways of clostridium butyricum for enhancing hydrogen production. Biotechnol Bioeng. 2013;110:338-42 31. Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrateproducing bacteria revealed by analysis of the butyryl-CoA:Acetate CoAtransferase gene. Environ Microbiol. 2010;12:304-314. 156 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii 32. Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099-2106. 33. Rabaey K, Rodriguez J, Blackall LL, et al. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007;1:9-18. 34. Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A. 2008;105:3968-3973. 35. Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. Biofilm and nanowire production leads to increased current in geobacter sulfurreducens fuel cells. Appl Environ Microbiol. 2006;72:7345-7348. 36. Vuong C, Kidder JB, Jacobson ER, Otto M, Proctor RA, Somerville GA. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J Bacteriol. 2005;187:2967-2973. 37. Wang YF, Tsujimura S, Cheng SS, Kano K. Self-excreted mediator from escherichia coli K-12 for electron transfer to carbon electrodes. Appl Microbiol Biotechnol. 2007;76:1439-1446. 38. Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91:915-923. 39. Voit EO, Almeida J, Marino S, et al. Regulation of glycolysis in lactococcus lactis: An unfinished systems biological case study. Syst Biol (Stevenage). 2006;153:286-298. 40. Masuda M, Freguia S, Wang YF, Tsujimura S, Kano K. Flavins contained in yeast extract are exploited for anodic electron transfer by lactococcus lactis. Bioelectrochemistry. 2010;78:173-175. 157 | P a g e Chapter 6 158 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Supplementary information Potentiosat Nitrogen Ag/AgCl reference electrode Graphite anode Platinum wire auxiliary electrode s Cathode Chamber Proton exchange membrane Sampling port Anode Chamber Supplementary Figure 1. Microbial fuel cell design implemented in the present study 159 | P a g e Chapter 6 Supplementary Table 1a: Anaerobic control of MFC growth experiment using F. prausnitzii as biocatalyst and riboflavin as redox mediater. The values shown are the consumption of glucose and acetate, the production of lactate, formate, butyrate and EPS, and the carbon and electron recoveries. Time (h) Glucose (mM) mM C mM eAcetate (mM) mM C mM eLactate (mM) 3 0.26 1.56 6.24 0.05 0.09 0.74 0.00 6 1.80 10.81 43.23 -1.49 -2.98 -23.82 0.00 9 2.42 14.52 58.08 0.25 0.50 4.00 0.00 12 2.49 14.94 59.77 0.07 0.15 1.19 0.00 16.5 2.96 17.76 71.05 -0.69 -1.39 -11.12 0.02 19 3.11 18.66 74.62 -0.92 -1.84 -14.69 0.07 22 3.48 20.86 83.46 -1.14 -2.28 -18.26 0.10 25 3.49 20.91 83.65 -1.34 -2.67 -21.36 0.10 mM C mM eFormate (mM) mM C mM eButyrate (mM) mM C mM eEPS (mg/ml) mM C 0.00 0.00 0.15 0.15 0.29 0.07 0.29 1.47 0.05 0.29 0.00 0.00 1.18 1.18 2.37 0.57 2.30 11.49 0.06 0.38 0.00 0.00 2.39 2.39 4.78 1.18 4.71 23.55 0.08 0.49 0.00 0.00 3.20 3.20 6.40 1.89 7.56 37.81 0.04 0.21 0.05 0.20 3.89 3.89 7.77 3.30 13.21 66.04 0.03 0.16 0.22 0.89 4.11 4.11 8.22 3.63 14.52 72.59 0.07 0.43 0.29 1.14 4.11 4.11 8.23 3.85 15.40 77.01 0.09 0.54 0.29 1.17 4.04 4.04 8.08 4.15 16.61 83.04 0.03 0.18 mM eRecoveries (%) Carbon Electron 1.16 1.50 1.98 0.85 0.65 1.71 2.15 0.70 53 59 28 25 56 59 74 77 90 91 93 93 87 84 88 86 160 | P a g e Electrogenic metabolism in Faecalibacterium prausnitzii Supplementary Table 1b: MFC-growth experiment using F. prausnitzii as biocatalyst and riboflavin as redox mediater with an applied potential of -0.35Volts vs Ag/AgCl . The values shown are the consumption of glucose and acetate, the production of lactate, formate, butyrate and EPS, and the carbon and electron recoveries. Time (h) Glucose (mM) mM C mM eAcetate (mM) mM C mM eLactate(mM) mM C mM eFormate (mM) mM C mM eButyrate (mM) mM C mM eEPS (mg/ml) mM C 3 0.91 5.49 21.95 0.26 0.52 4.15 0.00 0.00 0.00 0.10 0.10 0.21 0.07 0.29 1.44 -0.002 -0.01 6 1.20 7.20 28.82 0.11 0.23 1.83 0.00 0.00 0.00 0.38 0.38 0.76 0.28 1.14 5.69 0.003 0.02 9 1.72 10.31 41.22 0.01 0.03 0.22 0.00 0.00 0.00 1.34 1.34 2.68 1.03 4.14 20.69 0.02 0.13 12 2.07 12.40 49.62 0.26 0.52 4.15 0.00 0.01 0.04 2.69 2.69 5.38 1.75 6.99 34.96 0.01 0.06 16.5 2.36 14.17 56.68 0.04 0.07 0.57 0.00 0.00 0.01 3.07 3.07 6.13 2.22 8.88 44.41 0.00 0.01 19 2.42 14.50 58.02 -0.22 -0.45 -3.58 0.01 0.04 0.15 3.27 3.27 6.54 2.71 10.84 54.21 0.01 0.07 22 2.57 15.41 61.64 -0.03 -0.05 -0.43 0.04 0.11 0.43 3.41 3.41 6.83 2.65 10.58 52.91 0.01 0.09 mM eRecoveries (%) Carbon Electron -0.05 0.07 0.51 0.23 0.03 0.27 0.35 17 26 25 29 55 58 83 90 85 90 94 98 92 97 161 | P a g e Chapter 6 Supplementary Table 1c: MFC-growth experiment using F. prausnitzii as biocatalyst and riboflavin as redox mediater with an applied potential of +0.15 Volts vs Ag/AgCl . The values shown are the consumption of glucose and acetate, the production of lactate, formate, butyrate, EPS and current, and the carbon and electron recoveries. Time (h) Glucose (mM) mM C mM eAcetate (mM) mM C mM eFormate (mM) mM C mM eButyrate (mM) mM C mM eEPS (mg/ml) mM C mM eCurrent mM eRecoveries (%) Carbon Electron 162 | P a g e 3 0.68 4.09 16.36 -0.17 -0.35 -2.80 0.05 0.05 0.10 0.00 0.00 0.00 0.03 0.17 0.66 6 0.67 4.00 16.01 0.05 0.10 0.80 0.09 0.09 0.17 0.15 0.59 2.96 0.02 0.14 0.55 9 1.32 7.92 31.67 0.06 0.12 0.96 0.13 0.13 0.26 0.20 0.80 4.00 0.00 0.00 0.02 12 1.69 10.14 40.55 -0.11 -0.21 -1.68 0.31 0.31 0.62 0.54 2.17 10.85 -0.01 -0.06 -0.24 16.5 2.02 12.14 48.55 -0.09 -0.18 -1.43 0.93 0.93 1.86 1.00 4.00 20.00 -0.01 -0.04 -0.15 19 2.41 14.49 57.95 -0.34 -0.67 -5.39 1.54 1.54 3.08 1.80 7.20 36.00 0.00 0.02 0.08 22 2.49 14.97 59.86 -1.20 -2.39 -19.15 1.88 1.88 3.76 2.86 11.42 57.12 -0.01 -0.03 -0.14 25 2.89 17.31 69.26 -0.65 -1.30 -10.39 2.16 2.16 4.32 2.80 11.20 55.99 -0.01 -0.05 -0.18 0.09 0.38 0.92 2.08 4.91 5.98 6.86 7.53 5 4 23 30 13 19 24 26 40 40 58 56 76 71 72 69 Electrogenic metabolism in Faecalibacterium prausnitzii Supplementary Table 1d: MFC-growth experiment using F. prausnitzii as biocatalyst and riboflavin as redox mediater with an applied potential of +0.35 Volts vs Ag/AgCl . The values shown are the consumption of glucose and acetate, the production of lactate, formate, butyrate, EPS and current, and the carbon and electron recoveries.. Time (h) Glucose (mM) mM C mM eAcetate (mM) mM C mM eFormate (mM) mM C mM eButyrate (mM) mM C mM eEPS (mg/ml) mM C mM eCurrent mM eRecoveries (%) Carbon Electron 3 1.00 5.99 23.96 -0.08 -0.17 -1.34 0.06 0.06 0.13 0.00 0.00 0.00 6 1.16 6.95 27.80 0.09 0.17 1.40 0.32 0.32 0.65 0.22 0.89 4.43 9 1.24 7.45 29.82 0.10 0.19 1.53 1.08 1.08 2.15 0.65 2.59 12.97 12 1.63 9.79 39.15 0.17 0.33 2.66 2.11 2.11 4.23 1.46 5.85 29.24 15 2.13 12.76 51.04 0.29 0.59 4.72 2.69 2.69 5.39 1.69 6.75 33.74 18 2.29 13.72 54.88 0.15 0.31 2.48 2.86 2.86 5.71 1.89 7.57 37.86 24 2.36 14.18 56.71 0.14 0.29 2.29 3.01 3.01 6.01 2.15 8.61 43.07 -0.002 -0.01 -0.05 0.003 0.02 0.07 0.02 0.13 0.51 0.01 0.06 0.23 0.001 0.01 0.03 0.01 0.07 0.27 0.01 0.09 0.35 0.22 0.81 1.96 3.35 4.44 5.16 5.65 1 1 20 26 54 64 85 101 79 95 79 94 85 101 163 | P a g e Chapter 6 Supplementary Table 1e: MFC-growth experiment using F. prausnitzii as biocatalyst and riboflavin as redox mediater with an applied potential of +0.62 Volts vs Ag/AgCl . The values shown are the consumption of glucose and acetate, the production of lactate, formate, butyrate, EPS and current, and the carbon and electron recoveries. Time (h) Glucose (mM) mM C mM eAcetate (mM) mM C mM eFormate (mM) 3 0.71 4.23 16.94 0.23 0.47 3.73 0.09 6 1.40 8.42 33.69 0.30 0.60 4.79 1.44 9 1.84 11.01 44.04 0.67 1.35 10.77 3.14 12 2.82 16.89 67.57 0.42 0.84 6.71 4.15 16.5 2.99 17.93 71.71 0.11 0.21 1.70 4.24 19 3.16 18.96 75.85 0.18 0.35 2.81 4.30 22 3.25 19.50 78.00 0.22 0.45 3.60 4.29 25 3.30 19.81 79.23 0.11 0.21 1.70 4.20 mM C mM eButyrate (mM) mM C mM eEPS (mg/ml) mM C mM eCurrent mM eRecoveries (%) Carbon 0.09 0.18 0.00 0.00 0.00 0.03 0.16 0.66 1.44 2.88 0.80 3.22 16.09 0.04 0.22 0.89 3.14 6.27 1.48 5.91 29.57 0.03 0.19 0.78 4.15 8.31 2.42 9.68 48.39 0.03 0.18 0.71 4.24 8.49 2.84 11.38 56.89 0.01 0.04 0.15 4.30 8.60 2.94 11.76 58.80 0.01 0.06 0.24 4.29 8.58 2.93 11.71 58.54 0.00 0.01 0.03 4.20 8.41 2.91 11.65 58.23 0.01 0.06 0.25 0.90 1.74 2.72 3.74 5.01 5.66 6.38 7.02 17 32 65 78 96 114 88 100 89 101 87 100 84 99 81 95 Electron 164 | P a g e