* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NIPS April 1998 notebook
Survey
Document related concepts
Transcript
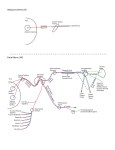
Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 The Lacrimal Gland and Its Veil of Tears Benjamin Walcott The secretory cells of the lacrimal gland produce a highly complex product of water, ions and proteins. At least five neurotransmitter receptors and three different second message systems are involved in controlling the different secretory processes of this highly sophisticated secretory epithelium. T he tear film that covers the anterior surface of the mammalian eye has a variety of constituents that are essential for the maintenance of the avascular transparent corneal epithelium. This film keeps the cornea wet, thus allowing gas B. Walcott is an Associate Professor in the Dept. of Neurobiology and Behavior at the State University of New York, Stony Brook, NY 11794–5230, USA. 0886-1714/98 5.00 @ 1998 Int. Union Physiol. Sci./Am.Physiol. Soc. exchange between the air and the epithelium. It cleans debris from the transparent surface, providing a clear optical path to the retina, and protects the ocular surface from invasion by bacteria and viruses. The tear film also provides essential metabolites such as retinol, which serves to preserve the transparent nature of the epithelium. The tear film is structurally complex with three distinct layers: a surface lipid layer (0.1–0.2 µm thick), a middle aqueous layer (7–8 µm thick), News Physiol. Sci. • Volume 13 • April 1998 “The tear film also provides essential metabolites such as retinol. . . .” 97 The nature of tear fluid The fluid secreted by the lacrimal glands is a complex solution of ions and proteins produced by two resident secretory cell populations: the plasma cells of the immune system and the acinar and duct cells of the secretory epithelium of the gland. The plasma cells are found in the interstitial spaces of the gland and migrate into it from lymphoid organs such as the gut-associated lymphoid tissue (GALT). These plasma cells secrete immunoglobin A (IgA) which is important in protecting the ocular surface from infection. The acinar cells of the secretory epithelium have three main functions: to synthesize and secrete a number of tear-specific proteins, to secrete water, and to transport the IgA secreted by the plasma cells from the interstitial compartment into the lumen of the gland. The lacrimal gland-specific proteins found at highest concentrations in the tears are lactoferrin, tear-specific prealbumin (TSP or lipocalin), and lysozyme (7). Other proteins occurring at lower concentrations are amylase, peroxidase, plasminogen activator, prolactin, epidermal growth factor (EGF), transforming growth factor-β (TGFβ), endothelin-1, and retinol. Lactoferrin, TSP, peroxidase, and lysozyme as well as IgA function 98 News Physiol. Sci. • Volume 13 • April 1998 to protect the cornea from viral and bacterial infections. This is important because the cornea is a wet, warm surface and thus is an ideal pathway for pathogens to invade the body and to affect the cornea. Retinol, which is derived from vitamin A, is necessary for the health of the cornea. The growth factors, TGF, EGF, and endothelin-1, are thought to be involved in the wound healing process in response to corneal abrasion or ulceration. The osmolarity of the lacrimal fluid is about 300 mosM and contains Na+ (128.7 mM), K+ (17 mM), Cl– (141.3 mM), and bicarbonate (HCO3–; 12.4 mM) (12). This fluid has about the same osmolarity as plasma but has lower Na+ (140 mM plasma) and higher K+ (4 mM plasma) and much higher Cl– (100 mM plasma). As is discussed later, the higher K+ and Cl– are a reflection of the way in which water is moved across the epithelium and into the gland lumen. Anatomy The lacrimal glands consist of a tubular secretory epithelium organized into lobes that drain into ducts; these ducts anastomose into larger ducts that finally drain onto the ocular surface. Associated with the secretory tubules are myoepithelial cells (which are thought to “squeeze” the secretory products down the tubules), fibroblasts (which produce the collagen and matrix that fill the interstitial regions), and occasional mast cells (which secrete histamine and heparin). In addition, there are B cells and T cells of the immune system as well as plasma cells normally scattered throughout the interstitium of the gland. As with most secretory epithelia, the cells of the secretory tubules (the acinar cells) are columnar with basally located nuclei and a large perinuclear Golgi apparatus. The duct cells are similarly organized, although they are more cuboidal in shape. The apical portion of the acinar and duct cells is filled with vesicles, which, in most cells, are not dense and therefore give the cell a “frothy” appearance in the light microscope. The base of the cells has an associated basement membrane, which is important in the polarization and function of the cell. The cells have a large junctional complex near the luminal pole that serves to mechanically attach the cells to each other and to couple them electrically and chemically. There are extensive gap junctions in this region, which consist of the connexins 26 and 32. Gap junctions can also be found outside of the junctional complex in some species such as the mouse. The high density of junctions in this gland suggests that acinar cells could be coupled both within and between secretory tubules. Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 “The growth factors. . . are thought to be involved in the wound healing process. . . .” and an inner mucus layer (30 µm thick). The mucus layer is associated with the microvilli of the corneal epithelial cells and becomes less dense toward the aqueous layer where the boundary is indistinct. In mammals, the mucus layer is produced by goblet cells in the cornea and conjunctiva, the aqueous layer by the lacrimal glands and other accessory glands, and the surface lipid layer by the meibomian glands and, in the case of many nonprimates, by the harderian glands as well. The most studied of these sources of the tear film are the lacrimal glands, which are the largest of these organs in mammals and are easily accessible. In rodents, for example, the extraorbital lacrimal gland is found under the skin on the lateral side of the face near the ear. In the rabbit, the gland is located within the orbit but is relatively easy to remove and is larger in size. Most physiological studies have used glands from either the mouse, rat, or rabbit to examine the control and mechanisms of secretion by this epithelium. This work is important in part because dysfunction of the lacrimal gland can lead to dry eye, which is a painful and potentially blinding condition. The lacrimal gland epithelium also is an elegant secretory tissue of multiple functions with complex control systems that can serve as a model for other secretory epithelia. The glands are innervated by both the sympathetic and parasympathetic divisions of the autonomic nervous system (8). The detailed pattern and nature of the innervation vary considerably among different species, but all have extensive numbers of cholinergic fibers, many of which also contain the neuropeptide vasoactive intestinal polypeptide (VIP), and fewer adrenergic fibers. The rat extraorbital lacrimal gland, for example, has a moderate density of large nonvaricose fibers, whereas the same gland in the mouse has a very dense pattern of small, highly varicose fibers. Birds have large numbers of adrenergic neurons and also substance P-containing neurons (14). In the rabbit and rat, fibers that are positive for the enkephalins, specifically Leu and Met enkephalin, also exist. Other neuropeptides have been reported in various lacrimal glands as well; for example, in the monkey, neuropeptide Y and calcitonin gene-related peptide are present. The innervation therefore is highly complex and is species specific in detail. The nerves that innervate the lacrimal glands come from autonomic ganglia. The parasympathetic postganglionic neural cell bodies are found in the pterygopalatine (sphenopalatine) ganglion as well as the ciliary ganglion. Sympathetic fibers originate in the superior cervical ganglion, and there is some innervation, probably sensory, from the trigeminal ganglion (13). The pathways from these ganglia to the gland vary significantly from species to species. Protein secretion As described earlier, a number of proteins are synthesized and secreted by the lacrimal gland acinar cells. The secretion of these proteins is stimulated by the neurotransmitters and neuropeptides found in the neurons that innervate the gland. The acinar cells, therefore, have receptors for acetylcholine (muscarinic M3) (9), VIP (types I and II), and norepinephrine (α1 and β) and presumably, in some cases, have receptors for peptides of the proenkephalin family as well as other peptides such as neuropeptide Y, adrenocorticotropic hormone (ACTH), and αmelanocyte-stimulating hormone (α-MSH). Immunocytochemical localization of M3 muscarinic acetylcholine receptors and of both VIP receptors shows that not all acinar cells possess these receptors. However, because the acinar cells are extensively coupled by gap junctions, second messengers produced by activation of those receptors, such as Ca2+ and inositol trisphosphate (IP3), could easily diffuse from stimulated cells to adjacent nonstimulated ones, causing them to become activated. As far as is known, all the membrane receptors are coupled to G proteins that in turn regulate the activity of the several second messenger systems found in many cells (Fig.1). The muscarinic acetylcholine receptors are linked to G proteins (Gs and Gq/11), which are coupled to phospholipase C, resulting, on activaNews Physiol. Sci. • Volume 13 • April 1998 “. . .a number of proteins are synthesized and secreted by the lacrimal gland acinar cells.” 99 Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 FIGURE 1. A flow diagram showing the various receptors found on the acinar cell surface, their relation to second messenger systems, and their ultimate effect on protein and water secretion. Many gaps in this scheme remain to be filled, for example, how the activity of protein kinase C (PKC) and protein kinase A (PKA) actually increases vesicle fusion. Note that ion channels (and thus water movement) are largely regulated by intracellular Ca2+ (Cai), which is increased by the action of both inositol trisphosphate (IP3) (in the ACh pathway) and cAMP [in the vasoactive intestinal peptide (VIP) pathway]. Enk, enkephalin; aADR, a-adrenoreceptor; ACTH, adrenocorticotropic hormone; AC, adenylate cyclase; DAG, diacylglycerol. 100 News Physiol. Sci. • Volume 13 • April 1998 acinar cells have segregated secretory pathways for certain secreted proteins and neurotransmitters have different stimulatory effects on these secretory pathways. It is also possible that different populations of cells in the same gland have different secretory products whose secretion can be differentially controlled, resulting in a different secretory product. This latter possibility is less likely because of the high degree of coupling of acinar cells by gap junctions and the ease with which second messenger molecules could diffuse from one cell to another. The secretion of proteins by the acinar cells involves vesicle fusion with the apical membrane and depends on membrane movement from intracellular structures such as the Golgi apparatus. To conserve membrane, there is an endocytotic process of internalization and intracellular processing of apical membrane, as seen in most secretory cells. In addition in the lacrimal gland acinar cells, there is endocytotic movement of membrane from the basolateral surface into the cell where it is processed. Whereas some of this basolateral membrane is used for apical secretion, particularly of the sIgA complex, a significant amount is cycled back to the basolateral surface after intracellular processing. This basolateral membrane traffic may have several important functions. It can be an important route by which certain molecules such as prolactin enter the acinar cells and exert their regulatory effect. In addition, stimulated acinar cells can express major histocompatability complex II molecules, a characteristic of antigen-presenting cells of the immune system. The basolateral membrane recycling, therefore, could be involved in antigen presentation as well as secretion of autoantigens (11). Understanding this process is important for Sjögren’s syndrome, for example, a dry eye disease in which an autoimmune response occurs involving lymphocytic infiltration of the lacrimal gland that results in destruction of some acinar tissue with loss of function. Transport of IgA Another major function of the lacrimal gland acinar cells is to move dimeric IgA from the interstitial fluid into the tear ducts and thus onto the ocular surface. IgA is produced by plasma cells resident in the lacrimal gland. There is a constant traffic of these plasma cells into the gland, as antibodies can be evoked by antigens either placed on the ocular surface or introduced into the animal through other means such as the gut. It is thought that most plasma cells arise in the GALT and then migrate to peripheral lymphoid organs such as the lacrimal glands. Most circu- Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 “. . .to move dimeric IgA from the interstitial fluid. . . onto the ocular surface.” tion, in increased production of IP3 and diacylglycerol (DAG) (4). IP3 induces the release of intracellular stores of Ca2+ and also is believed to open membrane Ca2+ channels. These ion channels are few in number and are small so that their detection by patch-clamp methods is difficult. The effect of these actions is to increase the intracellular Ca2+ levels transiently as shown by experiments using the Ca2+-sensitive dye fura 2. DAG activates several protein kinase C (PKC) isozymes, specifically α, ε, and δ, which will further stimulate secretion. VIP receptors are coupled to G proteins that activate adenylate cyclase. The activation of the adenylate cyclase increases adenosine 39,59-cyclic monophosphate (cAMP) levels, which in turn activates protein kinase A, which causes the phosphorylation of proteins that stimulate protein secretion (5). In addition, VIP causes an increase in intracellular Ca2+, presumably by means of second message systems that open Ca2+ channels in the plasma membrane. The increase in intracellular Ca2+ from both acetylcholine and VIP stimulation will increase the open time and thus the total conductance of the Ca2+-dependent K+ and Cl– channels, which are important in the secretion of water. α-Adrenergic agonists stimulate protein secretion by activating PKC but not through the intermediary action of IP3, Ca2+, or cAMP. The enkephalins have an inhibitory effect on protein secretion induced by either VIP or acetylcholine. These receptors are coupled to Gi proteins that inhibit the stimulatory activity of other G proteins on the adenylate cyclase and phospholipase C systems. Most of the preceding data are from studies that determined the secretion of either total protein or a specific protein such as peroxidase from gland fragments from a variety of different animals. Given the species variation in the nature of the innervation, perhaps not all of the mechanisms described occur in all animals. However, it is clear that all species seem to have both acetylcholine and VIP present and have receptors for both. An additional generalization is that in the lacrimal glands, for example, in rabbits, where there are adrenergic nerve fibers, activation of both sympathetic and parasympathetic neurons induces an increase in protein secretion. An issue then is the reason for such a complex innervation pattern with so many neurotransmitters and receptors if their sole function is to increase total protein secretion. A possible clue is seen in the rat secretion data, in which the ratio of a specific protein (peroxidase) to the total protein is different if stimulation is with carbachol (a muscarinic cholinergic agonist) or propranolol (a β-adrenergic agonist) (3). The possibility exists, then, that lating B cells that are destined to become plasma cells are specific for IgG with a minority being IgA. Therefore, there must be selective retention of the IgA cells within the lacrimal gland, although the precise mechanism is not known. The IgA produced by the resident plasma cells is secreted as a dimer, with the pair of antibody molecules linked by a protein called “J chain” that is also synthesized by the plasma cell. The complex is referred to as sIgA. For the sIgA to reach the glandular lumen, it must be transported through the acinar cells. To do this, the acinar cells produce a glycoprotein known as secretory component (SC) that functions as a “sacrificial receptor.” SC is found on the basolateral membranes of the acinar cells as well as in the membranes of the Golgi apparatus, secretory vesicles, and the rough endoplasmic reticulum. The sIgA complex has a high affinity for the SC and binds to it on the basolateral surface of the acinar cells. By the process of endocytosis, the SC-sIgA complex is internalized in vesicles and transported across the acinar cell to the apical surface. There, the vesicles fuse with the apical membranes of the acinar cells and the SC is cleaved into two parts, one of which is retained in the membrane and the other that remains attached to the now free sIgA complex. The majority of the IgA found in tears, therefore, consists of a dimer linked by J chain that has attached to it the soluble portion of the SC. This transport of sIgA is regulated by a variety of factors that affect the levels of SC synthesis in the acinar cells. Androgens, such as testosterone, increase “Androgens, such as testosterone, increase the levels of the SC and thus the rate of transport. . . .” Secretion of water One of the major secretory “products” of the lacrimal gland is water. This water is moved from the interstitial spaces of the gland into the lumen of the gland where it is mixed with the other secretory products. This water movement is accomplished by osmosis, which depends on the movement of particles (ions) from the acinar cells into the lumen (Fig. 2). Therefore, most studies News Physiol. Sci. • Volume 13 • April 1998 101 Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 FIGURE 2. Secretion of water by the acinar cells is by osmosis and is driven by the outward movement of ions, particularly Cl–. Neurotransmitter receptors are linked via second messenger systems to ion channels and regulate their permeability and thus the movement of water. the levels of the SC and thus the rate of transport (6). Understanding the role that androgens have in the normal functioning of the secretory acinar cells is important because over 90% of patients with Sjögren’s syndrome, a dry eye disease, are female. In addition to androgens, VIP induces a dose-dependent increase in SC production as do various lymphokines such as interleukin-1α and tumor necrosis factor-α. Isoproterenol (a β-adrenergic agonist) stimulates SC production but only in the presence of the hormone dihydrotestosterone. However, carbachol, an acetylcholine agonist, actually decreases SC synthesis, possibly by interfering with the production of cAMP. This reduction in SC due to carbachol is odd because acetylcholine is known to stimulate both protein and water secretion by these cells. Such an observation, however, does raise the interesting issue of different secretory pathways and their control and suggests that the complex innervation and second messenger pathways that exist in this gland could differentially regulate protein synthesis/secretion, water transport, and sIgA transport. Whereas sIgA is the dominant immunoglobulin in mammalian tears, smaller amounts of IgG and IgM are also produced by plasma cells in the gland. In birds, the major lacrimal gland, the harderian gland, has a very large population of plasma cells that secrete IgG and few that secrete IgA. IgG is not actively transported across the epithelium as is sIgA in mammals but rather diffuses between the epithelial cells. It seems likely that, in birds, large numbers of plasma cells are required to produce a significant concentration gradient across the epithelium so that diffusion of significant amounts of IgG can occur. In this gland, the plasma cells have cholinergic neurons among them and have muscarinic acetylcholine receptors on their surface (2). Activation of these receptors with carbachol increases intracellular Ca2+, which increases the rate of secretion of IgG above the basal level. Thus, in birds, there is neural modulation of the production of immunoglobulins, whereas, in mammals, there is modulation of the transport across the epithelium, but both systems serve to regulate the concentration of immunoglobulins in the tear fluid. “The apical domain is thought to contain water channels (aquaporin 5). . . .” 102 have examined the process of water movement indirectly by characterizing the membrane channels through which ions move in and out of the acinar cells. Similar to the salivary gland, the lacrimal gland has a distinction between the acinar cells that produce the bulk of the fluid and protein and the duct cells that modify the ionic composition of the fluid by retaining Na+. However, most physiological studies are not able to differentiate between these two cell types and most consider that these mechanisms take place in all the cells (Fig. 2). The acinar cell surface membrane is differentiated into basolateral and apical domain, which are separated by the junctional complex (Fig. 3). The apical domain is thought to contain water channels (aquaporin 5), which facilitate the movement of water across the epithelium. In addition, Cl– and K+ channels are present to allow the movement of solute across the epithelium. The basolateral membranes contain large numbers of Na+ pumps, the Na+-K+-ATPase, which actively move K+ into the cell and Na+ out of the cell, maintaining the usual gradients that are seen in all cells. It is this gradient (more Na+ outside and K+ inside) that provides the motive force for the movement of ions and water across the epithelium. In addition, there are several coupled transport systems (porters) driven by the concentration gradients created by the Na+ pump and by the activity of carbonic anhydrase. One cotransport system mediates the inward News Physiol. Sci. • Volume 13 • April 1998 movement of Na+ coupled to the outward flux of H+, and a second system affects the outward movement of bicarbonate ion (HCO 3–) as Cl– moves in (10). HCO 3– are produced by carbonic anhydrase in the cells and serve to buffer the lacrimal gland cells and fluid. The basolateral membranes also have ion channels, specifically for K+, Cl–, and Ca2+ as well as more general cation and anion channels. The Ca2+ channels are involved generally in the process of excitation/secretion coupling and, by affecting the permeability of other ion channels, indirectly regulate the movement of water. Because the number of Ca2+ ions that move is small and there are few channels, they have little direct effect on water movement themselves. The apical membrane of the cell, however, is believed to be rich in Cl– channels, which are Ca2+ sensitive. On activation of the cell, the raised intracellular Ca2+ will open the Cl– channels and that will allow an outward movement of Cl– into the lumen. Na+ will follow across the epithelium through the junctional complexes as well as through the cation channels in the acinar cells. This movement of ions into the lumen will osmotically drive the movement of water through the aquaporin channels into the lumen to maintain the osmotic balance. The movement of Cl– out of the apical membrane is dependent on the ion gradient of the Cl– across the cell membrane and on the relation of the membrane potential of the cell to the Cl– equi- Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 FIGURE 3. Diagram showing the major ion channels, coupled transporters (CT), and active transport systems (AT) responsible for the aqueous and ionic nature of the secretory product. Note that large numbers of Na+-K+ pumps (AT) in the basolateral membrane exist that create the ion gradients that allow the coupled transport systems to work. There are many types of K+ and Cl– channels, distinguished by their conductance, but most are regulated by Ca2+, and it is this process that regulates the movement of water across the epithelium. lins and water, which also are essential elements of the tear film. There are at least four neurotransmitter/neuropeptide receptors associated with the acinar cells that affect a number of different second message systems that, in turn, regulate the various secretory/transport processes. References 1. Brink, P. R., E. Peterson, K. Banach, and B. Walcott. Electrophysiological evidence for reduced water flow from lacrimal gland acinar epithelium of NZB/NZW F1 mice. In: Lacrimal Gland, Tear Film and Dry Eye Syndromes: Basic Science and Clinical Relevance, edited by D. A. Sullivan. New York: Plenum, 1997. 2. Brink, P. R., B. Walcott, E. Roemer, E. Grine, M. Pastor, G. J. Christ, and R. H. Cameron. Cholinergic modulation of immunoglobulin secretion from avian plasma cells: the role of calcium. J. Neuroimmun. 51: 113–121, 1994. 3. Bromberg, B. B., M. M. Cripps, and M. H. Welch. Peroxidase secretion by lacrimal glands from juvenile F344 rats. Invest. Ophthalmol. Visual Sci. 30: 562–568, 1989. 4. Dartt, D. A. Signal transduction and control of lacrimal gland protein secretion: a review. Curr. Eye Res. 8: 619–636, 1989. 5. Hodges, R. R., D. Zoukhri, C. Sergheraert, J. D. Zieske, and D. A. Dartt. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-tranducing components. Invest. Ophthalmol. Visual Sci. 38: 610–619, 1997. 6. Kelleher, R. S., L. E. Hann, J. A. Edwards, and D. A. Sullivan. Endocrine, neural, and immune control of secretory component output by lacrimal gland acinar cells. J. Immunol. 146: 3405–3412, 1991. 7. Kijlstra, A., and A. Kuizenga. Analysis and function of the human tear proteins. In: Lacrimal Gland, Tear Film, and Dry Eye Syndromes, edited by D. A. Sullivan. New York: Plenum, 1994, p. 299–308. 8. Matsumoto, Y., T. Tanabe, S. Ueda, and M. Kawata. Immunohistochemical and enzyme histochemical studies of peptidergic, aminergic and cholinergic innervation of the lacrimal gland of the monkey (Macaca fuscata). J. Auton. Nerv. Syst. 37: 207–214, 1992. 9. Mauduit, P., H. Jammes, and B. Rossignol. M3 muscarinic acetylcholine receptor coupling to PLC in rat exorbital lacrimal acinar cells. Am. J. Physiol. 264 (Cell Physiol. 33: C1550–C1560, 1993. 10. Mircheff, A. K. Lacrimal fluid and electrolyte secretion: a review. Curr. Eye Res. 8: 607–617, 1989. 11. Mircheff, A. K., J. P. Gierow, and R. L. Wood. Traffic of major histocompatibility complex class II molecules in rabbit lacrimal gland acinar cells. Invest. Ophthalmol. Visual Sci. 35: 3943–3951, 1994. 12. Rismondo, V., T. B. Osgood, P. Leering, M. A. Hattenhauer, J. L. Ubels, and H. F. Edelhauser. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. Contact Lens Assoc. Opthamol. J. 15: 222–228, 1989. 13. Vanderwerf, F., B. Baljet, M. Prins, and J. A. Otto. Innervation of the lacrimal gland in the cynomolgous monkey–a retrograde tracing study. J. Anat. 188: 591–601, 1996. 14. Walcott, B., P. A. Sibony, and K. T. Keyser. Neuropeptides and the innervation of the avian lacrimal gland. Invest. Opthalmol. Visual Sci. 30: 1666–1674, 1989. 15. Yiu, S. C., R. W. Lambert, P. J. Tortoriello, and A. K. Mircheff. Secretagogue-induced redistributions of Na,K-ATPase in rat lacrimal acini. Invest. Ophthalmol. Visual Sci. 32: 2976–2984, 1991. News Physiol. Sci. • Volume 13 • April 1998 “Patients with Sjögren’s syndrome have dry eyes and mouth. . . .” 103 Downloaded from http://physiologyonline.physiology.org/ by 10.220.32.247 on June 18, 2017 librium potential. As long as the membrane potential is below the Cl– equilibrium potential, Cl– will move out of the cell into the lumen and so will water. As the cell becomes depolarized, less Cl– will move and thus less water. If the membrane potential becomes equal to or depolarized above the Cl– equilibrium potential, then there will be no net outward Cl– or water movement. Because the movement of Cl– out of the cell will depolarize the membrane potential, the outward movement of K+ in the basolateral surface and apical domains is necessary to effectively provide a hyperpolarizing force and thus to maintain the membrane potential below the Cl– equilibrium potential. The permeability of both Cl– and K+ channels therefore regulates, and solute concentration gradient is the motive force for the movement of water across the epithelium. Because this movement results in both an efflux of K+ and an influx of Na+, the Na+-K+ pump must be present in high concentrations and must be active to counteract these fluxes and maintain a relatively constant gradient. Consistent with this is the observation that the stimulation of acinar cells with carbachol (an acetylcholine agonist) will move the Na+-K+ pumps from the Golgi membranes to the basolateral membranes of the cell, thereby increasing the effective movement of K+ in and Na+ out of the cells (15). This link between ion channels and their permeability and the movement of water may form the underlying cause of certain disease states in which lacrimal gland secretion of fluid is greatly reduced, resulting in dry eyes. Patients with Sjögren’s syndrome have dry eyes and mouth, and their lacrimal and salivary glands are heavily infiltrated with lymphocytes that have destroyed significant areas of secretory tissue. However, there are still areas of intact secretory acini that appear to be unable to secrete fluid. A possible explanation for this failure to transport water is suggested by measurements of membrane channels and the membrane potential in a mouse model of this disease, the NZB/NZW F1 female mouse. Cells from young, nondiseased animals have large numbers of active maxi-K+ channels and have membrane potentials on the order of –40 mV. Recordings from the acinar cells of older diseased animals, however, do not show active maxi-K+ channels and the membrane potential is only –5 to –10 mV, above the normal Cl– equilibrium potential. Thus these cells do not have a significant outward movement of Cl– on activation and therefore secrete little water, resulting in the dry eye condition (1). In summary, the lacrimal gland secretory epithelium synthesizes and secretes a number of specific proteins essential for the health of the cornea. In addition, it transports immunoglobu-