* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download isolation and characterization of membranes from the cells of maize
Action potential wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Cell encapsulation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Lipid bilayer wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Signal transduction wikipedia , lookup
Membrane potential wikipedia , lookup
Ethanol-induced non-lamellar phases in phospholipids wikipedia , lookup
Cytokinesis wikipedia , lookup
SNARE (protein) wikipedia , lookup
List of types of proteins wikipedia , lookup
J. Cell Sci. 45, 147-167 (1980)
Printed in Great Britain © Company of Biologists Limited igSo
ISOLATION AND CHARACTERIZATION OF
MEMBRANES FROM THE CELLS OF MAIZE
ROOT TIPS
ELIAS A-H. BAYDOUN AND D. H. NORTHCOTE
Department of Biochemistry, University of Cambridge, Cambridge, CBz iQW,
England
SUMMARY
A discontinuous sucrose density gradient was used to separate membrane fractions from
a homogenate of maize rcottips.Endoplasmic reticulum-, Golgi apparatus-, plasma membraneand mitochondria-rich fractions were identified by their enzymic characteristics and by
their appearance under the electron microscope. Maize roots were incubated in vivo with
D-[U-l4C]glucose, [Me-14C]choline chloride and diazotized [U-3H]sulphanilic acid. The
pattern of incorporation of radioactivity into the various membrane fractions was investigated.
Analyses of the polypeptide chains of the membrane fractions by SDS-polyacrylamide gel
electrophoresis showed that the mitochondria-rich fraction had a different pattern of polypeptides from that of the other membrane fractions. The results are discussed in relation to
the hypotheses of endomembrane flow and differentiation.
INTRODUCTION
The endomembrane system of eukaryotic cells includes the nuclear envelope, rough
and smooth endoplasmic reticulum, Golgi apparatus, plasma membrane and various
cytoplasmic vesicles (Northcote, 1971, 1974). Some of the components of the system
are joined by structural connexions and they all have a functional continuity in the
cytoplasm of eukaryotic cells (Morr6 & Ovtracht, 1977). It is now generally believed
that the endomembrane system is present in a dynamic state representing a flow of
membranes from the endoplasmic reticulum through the Golgi apparatus to the
plasma membrane (Bowles & Northcote, 1974; Morre, Kartenbeck & Franke, 1979).
In the last few years, attention has been directed to the separation of the endomembranes from plant cells. Several fractionation systems have been developed, but
most of them aim to separate only one component of the system from a total homogenate of cells (Powell & Brew, 1974; Hodges & Leonard, 1974). The difficulties met
in separating and identifying intact endomembranes from plant cells arise from the
presence of the cell wall and the lack of reliable markers for the different parts of the
endomembrane system (Quail, 1979). The first problem has been overcome for some
tissues by the use of protoplasts as starting material (Galbraith & Northcote, 1977).
The purpose of this study was to develop a simple and quick procedure to separate
membrane fractions from maize root tips. We avoided pelleting and re-suspension of
membranes to decrease the chance of their destruction.
Please address all correspondence to Professor D. H. Northcote at the above address.
148
E. A-H. Baydoun and D. H. Northcote
MATERIALS AND METHODS
Radioactive chemicals
D-[U-14C]glucose (sp. radioact. io-8 GBq/mmol), UDP-D-[U-"C]glucose (sp. radioact.
10-5 GBq/mmol), [Me-14C]choline chloride (sp. radioact. 2-2 GBq/mmol) and [U-3H]sulphanilic acid were purchased from the Radiochemical Centre, Amersham, Bucks., U.K.
[U-'H]sulphanilic acid was supplied as an impure solution (111 GBq) and was purified by
thin-layer chromatography in ethyl acetate/propan-2-ol/aq.NH, (sp.gr. o-88o) /water (6:4:2:1,
by vol.) on silica gel plates (20 cm x 20 cm, layer 2 mm; Macharey, Nagel und Co., Dilren,
FRG). The sp. radioact. of the purified material was o-i GBq/mmol.
Plant material
Seedlings of maize (Zea mays; var. Caldera) were grown under sterile conditions. The seeds
(coated with a copper-containing fungicide) were washed in sterile water, soaked in sterile
chloramphenicol solution (10 mg I."1) overnight, and germinated for 3-4 days in the dark at
25 °C as described by Harris & Northcote (1970). Harvesting and all subsequent procedures of
fractionation were carried out in the light at 4 °C.
Preparation of membrane fractions
Primary root tips (4-5 mm long) were excised with scissors and washed twice in the homogenization medium. Root tips (5 g fresh weight) were then suspended in 5 ml of the homogenization medium, chopped into small pieces and ground gently in a chilled mortar for
90-120 8, using a squashing action. The homogenization medium consisted of 8 % (w/w)
sucrose, 50 mM Tris-HCl buffer at pH 7-4, 1 mM EDTA, and o-i mM MgCla.
After filtration through 2 layers of muslin, the homogenate was centrifuged at 800 g for
10 min to remove starch, nuclei, cell wall fragments and unbroken cells. The volume of the
supernatant was made up to 12 ml by the addition of homogenization medium and immediately
layered on a discontinuous sucrose density gradient. This was prepared in a 38-ml cellulose
nitrate tube by layering in succession 4 ml of 45 % (w/w) sucrose and 5-5 ml each of 39, 34,
25 and 14 % (w/w) sucrose solutions, using a peristaltic pump. The sucrose solutions used to
make the discontinuous gradient contained all the components of the homogenization medium
except that the Tris-HCl buffer, pH 7-4, was at 10 mM. The tube was then centrifuged at
100000 g for 4 h and the particulate material at each sucrose interface was carefully collected
with a Pasteur pipette. The membrane fractions collected at the 14-25, 25-34, 34-39 a n d 3945 % interfaces are referred to as membrane fractions 1,2,3 ar>d 4 respectively, and all the material left in the tube after the collection of interfaces represents the remainder fraction (Fig. 1).
Marker enzyme assays
NADH-, and NADPH-cytochrome c reductases were assayed by following the reduction of
cytochrome c (^4^0 I8"5 mM"1 cm"1) (Shore & Maclachlan, 1975) using a Beckman Model 25
recording spectrophotometer. The effect of antimycin A (14 fig ml"1) on NADH-cytochrome c
reductase was also investigated. Succinate dehydrogenase activity was determined by following
spectrophotometrically the reduction of 2,6-dichlorophenol-indophenol (£«oo 21 mM"1 cm"1)
(Veeger, Der Vartanian & Zeylemaker, 1969). Latent IDPase was measured after storing the
membrane fractions at 4 °C for 4 days (Ray, Shininger & Ray, 1969). Mg*+-ATPase was
assayed at pH 6-5 (Leonard & Van Der Woude, 1976; Taussky & Shorr, 1953). UDPG:sterol
glucosyltransferase was assayed by a modification of the method of Lercher & Wojciechowski
(1976). The incubation was carried out at 30 °C for 30 min in a solution (0-4 ml) containing
membrane fraction (50 /tg protein), 50 mM Tris-HCl, pH 7-4, o-8 mM /?-mercaptoethanol,
10 mM MgCl,, o-8/tM UDP-D-[U-14C]glucose and 0-2% Triton X-100 with and without
0-5 mM /?-sitosterol. The reaction was started by the addition of the membrane fraction and
stopped by the addition of 6 ml of chloroform/methanol (3:2, v/v). After centrifugation, the
Membranes from maize root tips
149
supernatant was washed according to the method of Folch, Lees & Sloane Stanley (1957),
roto-evaporated and assayed for radioactivity. UDP-galactose:2V-acetylglucosamine galactosyltransferase was assayed as described by Palmiter (1969). Protein was estimated by the
method of Lowry, Rosebrough, Farr & Randall (1951) after precipitation of the membranes
with 18 % cold trichloroacetic acid. Bovine serum albumin was used as the standard.
Maize root tips + Homogenization medium
chopped into small pieces,
ground gently with a pestle
and mortar for 90—120 s
and filtered through 2
layers of muslin
Homogenate
centrifuged (800g, 4°C, 10 min)
Supernatant
800 o; pellet
layered on a discontinuous
sucrose gradient consisting
of 4 ml of 45% and 5-5 ml
each of 39, 34, 25 and 14%
(w/w) sucrose solutions.
Centrifuged (100000 c;, 4 °C, 4 h)
Membrane
fraction
8%
Density range
(amr1)
14%
Remainder]
fraction
14—25% interface-
-1-
•1058-1-109
25-34% interface-
-2-
•1-109—1-153
34—39% interface-
•3-
1-153-1-178
39—45% interface-
•4-
.1-178-1-210
25%
34%
39%
45%
Fig. 1. Scheme summarizing the procedure for the preparation of membrane fractions
from maize root tips.
Incubation of roots in vivo with diazotized [U-3H]sulphanilic acid
[U-3H]sulphanilic acid was converted to the diazonium salt (Berg & Hirsh, 1975). The
diazotization mixture contained [U-'H]sulphanilic acid (925 kBq), 2 /tmol HC1 and 50 /tl
(300 /imol) isoamyl nitrite. Maize seedlings (40-50) were arranged in a perforated plate so that
their root tips dipped into a Petri dish containing the diazotized [U-3H]sulphanilic acid
(925 kBq) dissolved in 5 ml of sterile water and were then incubated in the dark at 25 °C with
reciprocal shaking. After 60 min the roots were washed repeatedly with water to remove root
slime and non-covalently bound radioactivity, surface dried, excised, mixed with non-radioactive root tips, homogenized and fractionated.
150
E. A-H. Baydoun and D. H. Northcote
The various membrane fractions were suspended in the homogenization medium and pelleted
at 100 000 g and 4 °C for 30 min. The 800 g pellet and membrane pellets were suspended
in 18% (w/v) trichloroacetic acid. The remainder fraction was precipitated with 18% (w/v)
trichloroacetic acid. The insoluble material was washed 4 times with 18 % (w/v) trichloracetic
acid followed by acetone which was removed by heating in an oven at 60 °C for 5 min. It was
then dissolved in 50 fil of 1 M NaOH, neutralized with 1 M HC1, re-suspended in 1-9 ml of the
scintillation fluid and assayed for radioactivity.
Incubation of roots in vivo with v-[U-14C] glucose
Maize seedlings (30) were placed radially in circular groups (10 seedlings per group) in a
sterile Petri dish so that the tips of the roots were in contact. They were then incubated with
sterile D-[U-14C]glucose solution (10 fil, 37 kBq per root) by placing the solution at the point
of root contact. The roots were incubated in the dark at 25 °C for 45 min (Bowles & Northcote,
1974). At the end of the incubation, the slime and the incubation medium were removed and
the roots were washed repeatedly with sterile water and then surface dried. The root tips were
excised, mixed with non-radioactive root tips, homogenized and fractionated.
Analysis of membrane fractions prepared from roots incubated with D-[U-UC] glucose
in vivo
Membrane fractions that had been prepared from roots incubated with D-[U-14C]glucose
were divided into 3 equal aliquots, pelleted, washed with trichloroacetic acid and acetone, and
dried. The first aliquot was assayed for radioactivity. The second was dissolved in 72 % (w/w)
H2SO4 at 20 CC. After 4 h the acid was diluted to 3 % (w/w) H,SO4 and the preparation was
hydrolysed in an autoclave at 120 °C and 103 kN m" 1 for 1 h. The hydrolysates were neutralized with Amberlite IR-4B resin (CO3*~form), and roto-evaporated to dryness. The residues
were dissolved in water and applied to Whatman no. 1 paper and then run electrophoretically
in acetic acid/formic acid/water (4:1:45, by vol.) at pH 2 and 5 kV for 20 min (Harris &
Northcote, 1970). At the end of the run, neutral sugars and uronic acids remained near the
origin, while peptides and amino acids moved towards the cathode. These were cut from the
electrophoretogram and assayed for their radioactivity. The third aliquot was dissolved in
chloroform/methanol (3:2, v/v) to extract the lipids. The chloroform/methanol extract was
washed (Folch et al. 1957), roto-evaporated to dryness and assayed for radioactivity.
Incubation of roots in vivo with [Me-^Clcholine chloride
Maize seeds (100) were germinated under sterile conditions. After 2 days they were incubated
with 1110 kBq [Me-14C]choline chloride in 10 ml sterile water by addition of the radioactive
solution to the dish. After a further 48 h, the seedlings were removed and the roots were
thoroughly washed with water and then surface dried. The radioactive root tips were excised,
mixed with non-radioactive root tips, homogenized and fractionated. After pelleting the membrane fractions, lipids were extracted in chloroform/methanol (3:2, v/v). The extract was
washed (Folch et al. 1957), roto-evaporated to dryness and assayed for radioactivity.
To investigate the incorporation of [Afe-14C]choline into intact maize roots, radioactive
root tips (10) were homogenized at 4 °C, with a pestle and mortar in chloroform/methanol
(3:2, v/v; 5 ml) and the homogenate was stored at 4 °C overnight to extract the lipids and
centrifuged at ioooog for 30 min at 4°C. The lipid extract was decanted and the tissue
residue was further extracted with 3 ml of chloroform/methanol (3 :2, v/v). The pooled lipid
extracts were then washed (Folch et al. 1957), roto-evaporated to a small volume and analysed
by thin-layer chromatography. Thin-layer chromatography was carried out in chloroform/
methanol/water (65:25:4, by vol.) on silica-gel G plates (20 x 20 cm, layer 0-25 mm, without
gypsum; Camlab, Cambridge, U.K.). The tissue residue was washed several times with 80%
(v/v) methanol (Sharma, Babczinski, Lehle & Tanner, 1974), dried and assayed for radioactivity.
Membranes from maize root tips
151
Radioactivity determination
Radioactivity was determined using a Searle Mark III scintillation counter. Samples were
assayed for radioactivity for 10 min in a mixture of scintillation fluid made up of 2,5-diphenyloxazole (PPO), 4 g, i,2-Ws-(5-phenyloxazol-2-yl)benzene (POPOP), 0-075 g, Triton
X-ioo, 750 ml, in 1-5 1. of sulphur-free toluene. The efficiency for 14C was 90% and that for
3
H was 50 %.
SDS-polycrylamide gel electrophoresis
Membrane pellets were analysed on slab gels (Studier, 1973) using the system of Laemmli
(1970). Gels (15 %) were run at 200 V for approx. 5 h. After electrophoresis the proteins were
fixed in the gel with a mixture of methanol/acetic acid/water (9:2:9, by vol.), stained for
30 min with 0-125 % Coomassie brilliant blue made up in the fixer and destained by repeated
washing in a mixture of methanol/acetic acid/water (1:1:8, by vol). A mixture of marker
proteins (each 5 /tg) was run on each gel. The mixture consisted of lysozyme (mol. wt 14300),
creatine kinase (mol. wt 40000) and bovine serum albumin (mol. wt 68000).
Amino acid analysis
A sample of the 800 g pellet was dried in vacuo and was then hydrolysed with 6 M HC1 in
an evacuated sealed rube at 105 °C for 24 h. The hydrolysed sample was dried under vacuum
and then dissolved in 1 ml of 0-2 M citrate buffer, pH 2-2 (10-5 g citric acid, 11-7 g sodium
chloride, 3-5 ml Brj-35-(detergent) and 2-5 ml thiodiglycol I."1). Quantitative amino acid
analysis was performed using Rank Hilger Chromaspex J 180 Mark I amino acid analyser.
Electron microscopy
Membrane fractions were examined by negative staining with 2 % phosphotungstic acid
(adjusted with NaOH to pH 6-8) and they were prepared for thin sectioning, stained with
uranyl acetate and alkaline lead citrate and then examined in an AEI EM6B electron microscope at 60 kV (Brett & Northcote, 1975).
RESULTS
Distribution of enzyme activities
NADH-cytochrome c reductase, NADPH-cytochrome c reductase, succinate dehydrogenase, Mg2+-ATPase at pH 6-5, UDPG:sterol glucosyltransferase and UDPgalactose: 7V-acetylglucosamine galactosyltransferase were assayed immediately after
the preparation of fractions. Latent IDPase activity was assayed after storage of the
membranes at 4 °C for 4 days. The distribution of these enzyme activities is shown in
Table 1.
The presence of NADH-cytochrome c reductase and NADPH-cytochrome c
reductase is often used to identify the endoplasmic reticulum (Nagahashi & Beevers,
1978).
Fraction 1 contained 45-9 and 51-9% of the total activity of NADH- and NADPHcytochrome c reductases respectively. The activity of NADH-cytochrome c reductase
was found to be about 26 times higher than that of NADPH-cytochrome c reductase
(Table 1). When assayed in the presence of antimycin A, the activity of NADHcytochrome c reductase decreased markedly in some membrane fractions. It was inhibited by about 4-9, 9-5, 21-5 and 21-5% in membrane fractions i, 2, 3, and 4,
respectively.
I.
%
%
SP. act. (nmol mind' mg-'
protein)
Relative sp. act.
%
Succinate dehydrogenase
Total act. (nmol min-')
Sp. act. (nmol min-' rng-'
protein)
Relative sp. act.
%
Sp. act. (nrnol min-' mg-'
protein)
Relative sp. act.
NADPH-cytochrome c
reductase
Total act. (nmol rnin-')
+
NADH-cytochrome c
reductase ( antimycin A)
Total act. (nmol min-')
Sp. act. (nmol min-' mg-'
protein)
Relative sp. act.
%
NADH-cytochrome c
reductase (- antimycin A)
Total act. (nmol min-')
Homogenate 8 w g pellet
Fraction
2
Fraction
I
Fraction
3
Fraction
4
Remainder
fraction
Recovery
Lktribution of protein, and myme activities in subcellular fractions of maize root t@s obtained by discontinuous
sucrose density gradient centrifugation.
Protein
mg per 5 g fresh weight
Table
154
E. A-H. Baydoun and D. H. Northcote
IDPase has been shown to be a marker for the Golgi apparatus in plant cells, both
histochemically (Dauwalder, Whaley & Kephart, 1969) and biochemically (Ray et al.
1969; Coughlan & Evans, 1978). The highest latent IDPase specific activity was recovered in fraction 2. This fraction as well as the other isolated membrane fractions
did not show any activity when assayed for UDP-galactose:iV-acetylglucosamine
galactosyltransferase.
Table 2. Rough estimate of sterol in membrane fractions
Fraction
Homogenate
800 g pellet
Fraction 1
Fraction 2
Fraction 3
Fraction 4
Remainder fraction
Recovery
/tmol sterol mg" 1 protein
Total sterol, fimol
3-1
2-S
i-6
174-8
2-2
8-3
7-7
10-4
93-7
4-0
2-7
2-9
162
9-2
%
100
9-3
5-3
4-7
4-4
5-9
53-6
83-2
Endogenous sterols were rougly estimated by comparing the activity of UDPG: sterol
glucosyltransferase with added /?-sitosterol and that without added /?-sitosterol (Table 1)
using the following equation:
incorp" without /?-sitosterol
0-5 mM x .
——-—:
• :
—
-— :
i n c o r p " with p-sitosterol - i n c o r p n without /J-sitosterol
where 0-5 i t m is the final concentration of added /?-sitosterol in a volume of 0-4 m l containing
50 fig protein ( m e m b r a n e fraction).
Mg2+-ATPase, assayed at pH 6-5, has been demonstrated to be a plasma membrane
marker in plants (Galbraith & Northcote, 1977; Boss & Ruesink, 1979). The highest
specific activity of this enzyme was found in fraction 3 and the remainder fraction
contained high levels as well as IDPase. These activities could be due to non-specific
phosphatases contained in vacuoles which were broken during homogenization. The
high recovery of Mg2+-ATPase (155 %) is probably due to the dilution of an inhibitor
such as phosphate ions and is in agreement with results found in soybean suspension
cultures (Galbraith & Northcote, 1977). Hartmann, Fonteneau & Benveniste (1977)
showed that UDPG:sterol glucosyltransferase was located in the plasma membrane
of maize. When membrane fractions were assayed for this enzyme, the bulk of radioactivity incorporated from UDP-D-[U-14C]glucose was found in fractions 3 and 4,
but the highest specific activity occurred in fraction 3 and corresponded to that of
Mg2+-ATPase at pH 6-5. The enzymic activity was investigated with endogenous
sterols as well as with added /?-sitosterol. The addition of /?-sitosterol enhanced the
activity by 2-4-fold in the various membrane fractions. The high recovery (135 %)
could be due to dilution of an inhibitor or activation of the enzyme upon homogenization and fractionation.
Endogenous sterols were roughly estimated by comparing the incorporation of radioactivity with and without added /?-sitosterol (Table 2). The results showed that fraction
3 contained more sterols mg"1 protein when compared with other isolated fractions.
Membranes from maize root tips
155
Succinate dehydrogenase, which is localized in the inner mitochondrial membrane
(Janiszowska, Sobocinska & Kasprzyk, 1979), was found mainly in fraction 4 (73-5 %
of total activity). No activity was found in fractions 1 and 2.
These data suggest that fraction 1 was enriched in endoplasmic reticulum, fraction 2
in Golgi apparatus, fraction 3 in plasma membrane and fraction 4 in mitochondria.
Electron-microscopic investigations of the membrane fractions
800 g pellet. It was not possible to obtain electron micrographs of sufficient quality
from this pellet because of the large amounts of non-membranous material. Thin
sections showed that it was enriched in cell wall fragments. However, broken nuclei,
a few intact nuclei, plastids with starch grains, mitochondria and trapped membranes
were also present.
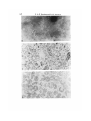
Fraction 1. Negative staining and thin sections of this fraction showed smooth and
some rough surface vesicular membranes of different sizes (Figs. 2, 3).
Fraction 2. On examination of negatively stained samples, these membranes showed
high proportions of individual Golgi cisternae (Fig. 4). They had characteristic
structures identical to those described by Brett & Northcote (1975). Thin sections
revealed the presence of structures similar to individual cisternae (Fig. 5). Vesicles of
various sizes were also present.
Fraction j . Examination of negatively stained and thin-sectioned material of this
fraction showed that it was rich in vesicles with smooth membranes. A few mitochondria were also present (Figs. 6, 7).
Fraction 4. Thin sections of this fraction showed enrichment in intact mitochondria.
A few broken mitochondria and vesicular material were also present (Fig. 8).
Distribution of radioactivity incorporated from diazotized \U-3H]sulphanilic acid
The distribution of radioactivity incorporated from diazotized [U-3H]sulphanilic
acid between the various membrane fractions is given in Table 3. Most of the radioactivity was found to be in the 800-g pellet. This could be because of the presence of
unbroken cells in this pellet. However, it could also be due to structural proteins and
entrapped plasma membrane within the cell wall fragments of the 800-g pellet. Among
the other membrane fractions the highest specific activity occurred in membrane
fraction 3 which had been shown by enzyme assays to be plasma membrane-rich.
Incorporation of [Me-uC]choline into maize root lipids
Roots were incubated with [Afe-14C]choline chloride for 48 h. Lipid extraction of
the roots removed 100% of the radioactivity. After Folch washing, all the radioactivity in the lipid phase co-chromatographed with authentic phosphatidylcholine
(Fig. 9). Most of the radioactive phosphatidylcholine was found in the endoplasmic
reticulum-rich fraction (Table 4).
Incorporation of X>-\U-uC]ghicose into maize roots
The incorporation pattern of radioactivity into neutral sugars and uronic acids,
amino acids and peptides and lipids in the various membrane fractions is shown in
E. A-H. Baydoun and D. H. Northcote
-^ \r
,&*'
rPoC&
*»
^
Membranes from maize root tips
157
Table 5. Most of the radioactivity was found in the %00-g pellet, owing to the presence
of cell wall fragments. The bulk of the radioactivity in this pellet, after hydrolysis, was
recovered in the neutral sugars and uronic acids which arose from cell wall polysaccharides. The ratio of incorporation of radioactivity into carbohydrate: protein increased in the sequence endoplasmic reticulum-, < Golgi apparatus-, < plasma membrane-rich fraction.
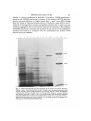
Electrophoretic patterns of the polypeptides of membrane fractions
The pattern of polypeptides produced on electrophoresis of the isolated membrane
fractions is shown in Fig. 10. A comparison of gel patterns revealed similarities and
differences between the various membrane fractions. The polypeptide pattern of the
mitochondria-rich fraction was unlike that of the other membrane fractions. The
plasma membrane-rich fraction showed some bands which were also present in the
mitochondria. This could be due to contamination by mitochondria. Electron
microscopy and marker enzyme determinations confirmed that the plasma membranerich fraction was contaminated with mitochondria.
Endoplasmic reticulum-, Golgi apparatus-, and plasma membrane-rich fractions
were similar. However, the Golgi apparatus-rich fraction showed some intense bands
in the mol. wt range of 40000 to 68000. The endoplasmic reticulum-rich fraction
contained a higher ratio of low molecular weight to high molecular weight polypeptides than the Golgi apparatus-rich fraction.
The 8oo-g- pellet (cell wall-rich) showed 2 characteristic bands of mobility close to
that of the bovine serum albumin marker and only few other bands which were
mainly of low molecular weight.
The cell wall of higher plants is known to contain a hydroxyproline-rich glycoprotein (Lamport & Northcote, i960). The cell wall-rich pellet was hydrolysed and
analysed for amino acid composition. The results (Table 6) showed that hydroxyproline accounted for 7-9 % of the total of mole % of amino acids in this pellet.
Fig. 2. Electron micrograph of fraction 1 (endoplasmic reticulum-rich) negatively
stained with 1 % sodium phosphotungstate, pH 6-8. x 10000.
Fig. 3. Electron micrograph of sectioned material from fraction 1 (endoplasmic
reticulum-rich) stained with uranyl acetate and alkaline lead citrate, x 10000.
Fig. 4. Electron micrograph of fraction 2 (Golgi apparatus-rich) negatively stained
with 1 % sodium phosphotungstate, pH 6-8. Arrows show structures which may
represent individual cisternae. x 10000.
Fig. 5. Electron micrograph of sectioned material from fraction 2 (Golgi apparatusrich) stained with uranyl acetate and alkaline lead citrate. Arrows show structures
which may represent individual cisternae. x 16000.
E. A-H. Baydoun and D. H. Northrnre
' • > : .
8
tJ
"
jt:t»ty-.
rw-A
Membranes from maize root tips
Table 3. Distribution of radioactivity incorporated from diazotized [U-3H]sulphanilic
acid into various membrane fractions of maize roots
Fraction
Homogenate
800 g pellet
Endoplasmic reticulumrich (Fraction 1)
Golgi apparatus-rich
(Fraction 2)
Plasma membrane-rich
(Fraction 3)
Mitochondria-rich
(Fraction 4)
Remainder fraction
Recovery
Total act.,
io~° x cpm
/o
1016-5
638-8
ioo-o
628
21-9
Sp. radioact.,
Relative sp.
10- 3 x cpm mg" 1 protein radioact.
i-oo
2-2
I7-S
87-5
3-8
O-22
39-4
3"9
12-7
o-73
62-7
62
45-8
262
85-i
8-4
25-8
1-48
8I-I
8-o
91-5
2-3
0-13
—
—
501
—
Roots were incubated with diazotized [U-'H]sulphanilic acid for 1 h in vivo, and then
homogenized and fractionated (Fig. 1). Radioactivity precipitated by trichloroacetic acid was
determined.
10 r
o
x
a
Front
I
4
8
12
Distance from origin (cm)
.1
16
Fig. 9. Thin-layer chromatography of the radioactive lipids extracted from maize roots
incubated in vivo with [Me-14C]choline chloride. Roots were incubated with
[Afe-14C]choline chloride for 48 h in vivo. Lipids were extracted with chloroform/
methanol (3 :2, v/v). The lipids were chromatographed on silica gel thin-layer plates,
which were then sliced and assayed to determine the distribution of radioactivity.
Fig. 6. Electron micrograph of fraction 3 (plasma membrane-rich) negatively stained
with i % sodium phosphotungstate, pH 6-8. x 10000.
Fig. 7. Electron micrograph of sectioned material from fraction 3 (plasma membranerich) stained with uranyl acetate and alkaline lead citrate, x 10000.
Fig. 8. Electron micrograph of sectioned material from fraction 4 (mitochondria-rich)
stained with uranyl acetate and alkaline lead citrate, x 10000.
i6o
E. A-H. Baydoun and D. H. Northcote
DISCUSSION
The discontinuous gradient described in this work provided a partial separation of
several enzyme activities and therefore of different membrane fractions. The identification of these membrane fractions was based on marker enzyme enrichment as well
as on morphological criteria derived from electron-microscopic investigation.
Fraction i (density < r i g m l " 1 ) was identified as endoplasmic reticulum-rich
Table 4. Distribution of radioactivity incorporated from [Me-uC]choline
various membrane fractions of maize roots
Sp. radioact.,
Relative sp.
10- 3 x cpm mg" 1 protein radioact.
Total act.,
io~ s x cpm
Fraction
Homogenate
800 g pellet
Endoplasmic reticulumrich (Fraction 1)
Golgi apparatus-rich
(Fraction 2)
Plasma membrane-rich
(Fraction 3)
Mitochondria-rich
(Fraction 4)
Remainder fraction
Recovery
chloride into
687-2
28-8
308-6
ioo-o
n-6
i-oo
4-2
4-i
44-9
48-4
0-35
4-18
1576
229
39-5
3-4i
53-9
7-8
23-4
2-O2
86-4
126
19-2
I-6 5
34-5
—
97H
5-°
N.D.*
—
N.D.
—
• N . D . not determined
Roots were incubated with [Me-14C]choline chloride for 48 h in vivo, and then homogenized
and fractionated (Fig. 1). Lipids were extracted with chloroform/methanol (3:2, v/v) and
assayed for radioactivity.
Table 5. Relative amounts of radioactivity incorporated from T>-\U-uC]glucose into
carbohydrate, protein and lipid components in various membrane fractions of maize roots
Radioactivity
Radioactive
component
800 g
pellet
Neutral sugars
52-o
& uronic acids
Amino acids
18-4
& peptides
Lipid extract
4-i
Total cpm
64070
Recovery
74-5
Endoplasmic
reticulum-rich
(Fraction 1)
Plasma
MitochondriaGolgi
rich
apparatus-rich membrane-rich
(Fraction 3)
(Fraction 4)
(Fraction 2)
I5-7
28-1
39-3
3349
37-i
28-1
24-0
34-6
19-1
8-9
18410
72-2
9-6
12870
i8- 4
10070
71-2
14
38670
75-3
78-1
Roots were incubated with D-[U- C]glucose for 45 min in vivo and then homogenized and
fractionated (Fig. 1). Membrane pellets were analysed as described in Materials and methods.
Membranes from maize root tips
l6i
fraction. It showed enrichment in antimycin A-insensitive NADH-cytochrome c
reductase and NADPH-cytochrome c reductase. In the absence of EDTA, the endoplasmic reticulum sediments at a higher density than r i g ml"1 since EDTA brings
about the release of ribosomal subunits (Green & Northcote, 1979). EDTA was included in the homogenization medium and gradient solutions that we used to prevent
membrane aggregation and to ensure better separation of membranes. The inhibition
of NADH-cytochrome c reductase with antimycin A was very low in the endoplasmic
reticulum-rich fraction as compared with the mitochondria-rich fraction which
showed maximum inhibition.
10
11
12
Mol. wt
68000
40000
.14300
+ve
Fig. 10. SDS-polyacrylamide gel electrophoresis of the isolated membrane fractions.
Tracks 1 and 2; mitochondria-rich fraction. Tracks 3 and 4; plasma membrane-rich
fraction. Tracks 5 and 6; Golgi apparatus-rich fraction. Tracks 7 and 8; endoplasmic
reticulum-rich fraction. Tracks 9 and 10: 8oo-|* pellet. Approx. 150 fig of protein
were applied per track. Tracks 11 and 12; protein markers with molecular weights as
indicated. Membrane fractions were isolated from maize roots as shown in Fig. 1.
After pelleting, membranes were analysed on a 15 % gel at 200 V for approx. 5 h.
Staining was with Coomassie brilliant blue.
162
E. A-H. Baydoun and D. H. Northcote
Mitochondria are known to contain 2 activities of NADH-cytochrome c reductase.
The first, which is located in the outer membrane, is antimycin A insensitive (Douce,
Mannella & Bonner, 1973) and similar to that of the activity found in the endoplasmic
reticulum. The second is antimycin A-sensitive and is located in the inner mitochondrial membrane (Lord, Kagawa, Moore & Beevers, 1973; Sparace & Moore,
1979). The significant inhibition of NADH-cytochrome c reductase by antimycin A
in fractions 3 and 4 could be due to broken mitochondria. No detergents were used
in the enzyme assay.
Table 6. Amino acid composition of the 800-g pellet
Amino acid
Mol<Yo
Amino acid
Hyp
Asp
Thr
Ser
Glu
Pro
Gly
Ala
Val
7'9
8-S
5-S
6-o
9-8
6-3
Met
He
Leu
Tyr
Phe
His
Lys
Arg
—
9-2
10-3
6-3
Mol %
i-o
3-8
69
2-0
2-8
2-6
5-6
S-2
The 800-g pellet was hydrolysed with 6 M HC1 under vacuum ai: 105 °C for 24 h. The
hydrolysed sample was dried, dissolved in citrate buffer and assayed for amino acid composition.
Fraction 2 was identified as the Golgi apparatus-rich fraction because of its association with IDPase. This fraction also showed high activities of antimycin A-insensitive
NADH-cytochrome c reductase and NADPH-cytochrome c reductase which could
result from contamination with membrane vesicles derived from endoplasmic
reticulum, or the presence of the enzymes as components of the Golgi apparatus.
Recent studies (Hino, Asano, Sato & Shimizu, 1978; Hino, Asano & Sato, 1978)
have shown that NADH- and NADPH-cytochrome c reductases are localized in the
Golgi fraction and their presence could arise from membrane flow between the membranes of the endoplasmic reticulum and the Golgi apparatus. Howell, Ito & Palade
(1978) reported evidence for the occurrence of NADPH-cytochrome c reductase in
isolated Golgi fractions from rat liver. This was further supported by immunological
studies (Ito & Palade, 1978).
Identification of isolated Golgi apparatus from plants is chiefly based on their
morphology, which is so characteristic that it serves as a reliable marker (Northcote,
1971, 1974). The secretory activity of the Golgi apparatus changes during the cell
cycle in maize root tips (Mollenhauer & Mollenhauer, 1978). The great difficulty in
isolating intact dictyosomes from plant tissue as distinct from animal tissue lies in the
method of homogenization. Plant dictyosomes become unstacked to produce individual cisternae after the comparatively rigorous homogenization required to break
open the cell walls. The method most commonly used for overcoming the problem
has been to include glutaraldehyde in the homogenization medium as a membrane
Membranes from maize root tips
163
fixative (Harris & Northcote, 1971; Bowles & Northcote, 1972). Although this treatment inactivates many enzymes (Ray, Eisinger & Robinson, 1976), the preservation of
subcellular organelles is good. The treatment of pea roots with cellulase and the inclusion of polyethylene glycol in the homogenization medium prior to homogenization
(Brett & Northcote, 1975) enabled intact dictyosomes to be isolated. Powell & Brew
(1974) isolated intact dictyosomes from onion stems by chopping the stems with razor
blades, followed by homogenization in an all-glass Potter-Elvehjem homogenizer. The
homogenization medium contained 10 mM MgCl2, which may result in membrane
aggregation.
In the present work, characteristic individual cisternae but not dictyosomes were
found. This could be due to the homogenization method (pestle and mortar) and also
to the presence of EDTA in the homogenization medium which will cause membrane
disaggregation because of the removal of divalent cations.
UDP-galactose: ./V-acetylglucosamine galactosyltransferase has been used as a
reliable biochemical marker for the Golgi apparatus especially in animal tissues (Morre^
Yunghans, Vigil & Keenan, 1974). It has been reported to be present in the Golgi
membrane of onion stems (Powell & Brew 1974) and of the vegetative tissue of the
brown alga Fucus serratus (Coughlan & Evans, 1978). We found no activity in any of
the isolated membrane fractions.
The highest specific activity of Mg2+-ATPase at pH 6-5 was found in fraction 3.
This fraction was identified as plasma membrane-rich, in agreement with results obtained for sugar cane cell suspension (Thorn, Laetsch & Maretzki, 1975), soybean
protoplasts (Galbraith & Northcote, 1977) and maize roots (Leonard & Van Der Woude,
1976). However, the activity of the Mg2+-ATPase, present in all the membrane
fractions we examined, was not enhanced by the addition of 50 mM K+, which contrasts with some other results (Leonard, Hansen & Hodges, 1973; Lurie & Hendrix,
1979), but agrees with those of Boss & Ruesink (1979). It was also not inhibited by
the addition of o-i mM diethylstilbestrol. Balke & Hodges (1979) reported diethylstilbestrol as a specific inhibitor for the plasma membrane-associated ATPase.
The plasma membrane-rich fraction showed enrichment in the activity of
UDPG: sterol glucosyltransferase. Our results are different from those of Lercher &
Wojciechowski (1976) and Bowles, Lehle & Kauss (1977), who suggested that the
enzyme was a convenient marker for the Golgi apparatus-rich fraction. Other data
obtained for eukaryotic cells showed that glucosyltransferase activity could be found
in the mitochondria (Ongun & Mudd, 1970) and in the microsomal fraction (Martin
& Thorne, 1974). Thus, the role of UDPG:sterol glucosyltransferase as a specific
marker enzyme is questionable.
In comparison with other membrane fractions, the plasma membranes are rich in
sterols (Keenan, Leonard & Hodges, 1973). From our results, a rough estimation of
sterol in the various isolated membrane fractions showed that the plasma membranerich fraction had the highest content. A high sterol concentration has been used as a
chemical marker to identify the plasma membrane in plants (Hodges & Leonard,
1974; Hartmann & Benveniste, 1978). However, chemical constituents are not sufficiently restricted to any single type of membrane to be used as unambiguous markers.
164
E. A-H. Baydoun and D. H. Northcote
Fraction 4 identified as the mitochondria-rich fraction showed enrichment in succinate dehydrogenase. This is in good agreement with other observations on plant
tissues (Williamson, Morre & Jaffe, 1975). Succinate dehydrogenase activity was
absent from the endoplasmic reticulum- and Golgi apparatus-rich fractions, but was
found in both the plasma membrane- and mitochondria-rich fractions. This was
probably due to contamination between these 2 fractions, as indicated by electron
microscopy. Contamination was possible because of the similarity of mitochondrial
and plasma membrane densities. In a preparation of the plasma membrane from
maize roots, Leonard & Van Der Woude (1976) removed mitochondria from the
homogenate by a low-speed differential centrifugation step (13 000 g). When this
method was attempted in our work, it resulted in major losses of plasma membrane
vesicles. Plasma membrane vesicles may also be lost by selective entrapment in the cell
wall residue during homogenization because of the close association between the
plasma membrane and the cell wall (Bailey & Northcote, 1976).
In our work, although the various isolated membrane fractions showed enrichment
in their specific marker enzymes, marker enzyme activity was also observed in other
membrane fractions. If all endomembranes are functionally interconnected, then the
presence of an enzyme on several components of the endomembrane system is to be
expected. However, the results may not only be explained by differentiation of intracellular membranes, but also by contamination between the different membrane fractions during their isolation.
Diazotized sulphanilic acid has been used as a probe for identifying the plasma
membrane in animal (Berg & Hirsh, 1975) and plant cells (Galbraith & Northcote,
1977). With maize roots, the radioactivity associated with the remainder fraction was
very small, indicating that the compound did not penetrate the cells to label the
internal proteins and membranes. Among the particulate fractions, although the
plasma membrane-rich showed the highest specific radioactivity, there were significant
amounts of radioactivity found in the mitochondria-rich fraction. This could be due
to contamination since plasma membrane and mitochondria were difficult to separate.
Although the result of this work is in good agreement with that of Galbraith &
Northcote (1977), the cell wall remains a serious obstacle in using diazotized sulphanilic
acid as a probe for labelling the plasma membrane of intact maize root cells in vivo as
distinct from protoplasts.
Phosphatidylcholine has been found to be the major structural phospholipid in the
eukaryotic cellular membranes (Phillips & Butcher, 1979). Incorporation of radioactive choline into the various isolated membrane fractions confirmed the results of
Dykes, Kay & Harwood (1976) that radioactive choline was incorporated only into
phosphatidylcholine during the first 48 h of germination in soybean. The endoplasmic
reticulum has been identified as the major site for phospholipid synthesis in various
plant and animal tissues (Lord, 1975; Jelsema & Morre, 1978; Quinn & Williams,
1978). However, the Golgi system has also been suggested to be involved in phospholipid synthesis (Montague & Ray, 1977). In our work, although the total activity
found in the endoplasmic reticulum was higher than that of the Golgi apparatus-rich
fraction, their specific activities were similar. The distribution of radioactivity also
Membranes from maize root tips
165
showed a decrease in the order endoplasmic reticulum- > Golgi apparatus- > plasma
membrane-rich fraction among the various isolated components of the endomembrane
system. This could be due to contamination among the isolated fractions or it could
result from the transport of synthesized phosphatidylcholine from the endoplasmic
reticulum-rich fraction to other membrane fractions. It has been proposed that the
Golgi apparatus is involved in the transformation of membranes of the endoplasmic
reticulum to the plasma membrane (Northcote, 1974; Moire", 1975).
Radioactive glucose was incorporated into the various chemical substances contributing to membrane composition and into the material within the lumen of the
membranes. The Golgi apparatus-rich fraction showed an intermediary position in
the distribution of radioactivity as compared to the endoplasmic reticulum- and
plasma membrane-rich fractions.
The characteristic patterns of the polypeptide chains in the various isolated fractions were compared using SDS-polyacrylamide gel electrophoresis. Although the
mitochondria-rich fraction was distinct from the other membrane fractions, the endoplasmic reticulum-rich fraction, the plasma membrane-rich fraction and the Golgi
apparatus-rich fraction resembled one another. These results are consistent with the
data published by other workers (Fleischer & Fleischer, 1970; Hodson & Brenchley,
1976). This similarity between the polypeptide chains of various membrane fractions
may again be explained by contamination between the membrane fractions or, it may
represent intra-cellular membrane turnover and differentiation (Northcote, 1979).
REFERENCES
BAILEY, D. S. & NORTHCOTE, D . H . (1976). Phospholipid composition of the plasma membrane
of the green alga, Hydrodictyon africanum. Biochem. J. 156, 295-300.
BALKE, N. E. & HODGES, T . K. (1979). Inhibition of adenosine triphosphatase activity of the
plasma membrane fraction of oat roots by diethylstilbestrol. PL PhysioL, Lancaster 63,
48-52.
BERG, H. C. & HIRSH, D . (1975). Synthesis of diazotized [35S]sulfanilic acid of high specific
activity: a label for the outer surface of cell membranes. Analyt. Biochem. 66, 629-631.
Boss, W. F. & RUESINK, A. W. (1979). Isolation and characterization of concanavalin Alabelled plasma membranes of carrot protoplasts. PL Physiol., Lancaster 64, 1005-1011.
BOWLES, D. J., LEHLE, L. & KAUSS, H. (1977). Glucosylation of sterols and polyprenolphos-
phate in the Golgi apparatus of Pliaseolus aurcus. Planta 134, 177-181.
BOWLES, D. J. & NORTHCOTE, D. H. (1972). The sites of synthesis and transport of extracellular polysaccharides in the root tissue of maize. Biochem. J. 130, 1133-1145.
BOWLES, D. J. & NORTHCOTE, D . H. (1974). The amounts and rates of export of polysaccharides
found within the membrane system of maize root cells. Biochem. J. 142, 139-144.
BRETT, C. T . & NORTHCOTE, D . H. (1975). The formation of oligoglucans linked to lipid
during synthesis of /?-glucan by characterized membrane fractions isolated from peas.
Biochem. J. 148, 107-117.
COUGHLAN, S. & EVANS, L. V. (1978). Isolation and characterization of Golgi bodies from
vegetative tissue of the brown alga Fucus serratus. jf. exp. Bot. 29, 55-68.
DAUWALDER, M., WHALEY, W. G. & KEPHART, J. E. (1969). Phosphatase and differentiation of
the Golgi apparatus. J. Cell Sci. 4, 455-497.
DOUCE, R., MANNELLA, C. A. & BONNER, W. D. (1973). The external N A D H dehydrogenases
of intact plant mitochondria. Biochim. biophys. Acta 29Z, 105-116.
DYKES, C. W., KAY, J. & HARWOOD, J. L. (1976). Incorporation of choline and ethanolamine
into phospholipids in germinating soya bean. Biochem. J. 158, 575-581.
166
E. A-H. Baydoun and D. H. Northcote
FLEISCHER, B. & FLEISCHER, S. (1970). Preparation and characterization of Golgi membranes
from rat liver. Biochim. biophys. Acta 219, 301-319.
FOLCH, J., LEES, M. & SLOANE STANLEY, G. H. (1957). A simple method for the isolation and
purification of total lipids from animal tissues. J. biol. Chem. 232, 497-509.
GALBRAITH, D. W. & NORTHCOTE, D. H. (1977). The isolation of plasma membrane from
protoplasts of soybean suspension cultures. J . Cell Sci. 24, 295-310.
GREEN, J. R. & NORTHCOTE, D. H. (1979). Location of fucosyl transferases in the membrane
system of maize root cells. J. Cell Sci. 40, 235-244.
HARRIS, P. J. & NORTHCOTE, D . H. (1970). Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem. J. 120, 479-491.
HARRIS, P. J. & NORTHCOTE, D. H. (1971). Polysaccharide formation in plant Golgi bodies.
Biochim. biophys. Acta 237, 56-64.
HARTMANN, M. A. & BENVENISTE, P. (1978). Sterol biosynthetic capability of purified membrane fractions from maize coleoptiles. Phytochemistry 17, 1037-1042.
HARTMANN, M. A., FONTENEAU, P. & BENVENISTE, P. (1977). Subcellular localization of sterol
synthesizing enzymes in maize coleoptiles. PI. Sci. Lett. 8, 45-51.
HINO, Y., ASANO, A. & SATO, R. (1978). Biochemical studies on rat liver Golgi apparatus. II.
Further characterization of isolated Golgi fraction. ,7. Biochem., Tokyo 83, 925-934.
H I N O , Y., ASANO, A., SATO, R. & SHIMIZU,
S. (1978). Biochemical studies of rat liver
Golgi apparatus. I. Isolation and preliminary characterization. J. Biochem., Tokyo 83, 909923HODGES, T . K. & LEONARD, R. T . (1974). Purification of a plasma membrane-bound adenosine
triphosphatase from plant roots. Meth. Enzym. 32, 392-406.
HODSON, S. & BRENCHLEY, G. (1976). Similarities of the Golgi apparatus membrane and the
plasma membrane in rat liver cells. J. Cell Sci. 20, 167-182.
HOWELL, K. E., ITO, A. & PALADE, G. (1978). Endoplasmic reticulum marker enzymes in
Golgi fractions - What does this mean? J. Cell Biol. 79, 581-589.
ITO, A. & PALADE, G. (1978). Presence of NADPH-cytochrome P-450 reductase in rat liver
Golgi membranes. Evidence obtained by immunoadsorption method. J. Cell Biol. 79,
590-597.
JANISZOWSKA, W., SOBOCINSKA, E. & KASPRZYK, Z. (1979). Distribution of different forms of
sterols in three subcellular fractions of Calendula officinalis leaves. Phytochemistry 18,
427-430.
JELSEMA, C. L. & MORRE, D. J. (1978). Distribution of phospholipid biosynthetic enzymes
among cell components of rat liver. J. biol. Chem. 253, 7960-7971.
KEENAN, T . W., LEONARD, R. T . & HODGES, T . K. (1973). Lipid composition of plasma
membranes from Avena sativa roots. Cytobios 7, 103-112.
LAEMMLI, U. K. (1970). Cleavage of structural proteins during the assembly of the head of
bacteriophage T 4 . Nature, Land. 227, 680-685.
LAMPORT, D . T . A. & NORTHCOTE, D . H . (i960). Hydroxyproline in primary cell walls of
higher plants. Nature, Lond. 188, 665-666.
LEONARD, R. T., HANSEN, D. & HODGES, T . K. (1973). Membrane-bound adenosine triphos-
phatase activities of oat roots. PL Physiol., Lancaster 51, 749—754.
LEONARD, R. T . & VAN DER WOUDE, W. J. (1976). Isolation of plasma membranes from corn
roots by sucrose density gradient centrifugation. An anomalous effect of Ficoll. PI. Physiol.,
Lancaster 57, 105-114.
LERCHER, M. & WOJCIECHOWSKI, Z. A. (1976). Localization of plant UDP-glucose: sterol
glucosyl transferase in the Golgi membranes. PI. Sci. Lett. 7, 337-340.
LORD, J. M. (1975). Evidence that phosphatidylcholine and phosphatidylethanolamine are
synthesized by a single enzyme present in the endoplasmic reticulum of castor-bean endosperm. Biochem. J. 151, 451-453.
LORD, J. M., KAGAWA, T., MOORE, T . S. & BEEVERS, H. (1973). Endoplasmic reticulum as the
site of lecithin formation in caster bean endosperm. J. Cell Biol. 57, 659-667.
LOWRY, O. H., ROSEBROUGH, N . J., FARR, A. L. & RANDALL, R. J. (1951). Protein measure-
ment with the Folin-phenol reagent. J. biol. Chem. 193, 265-275.
LURIE, S. & HENDRIX, D . L. (1979). Differential ion stimulation of plasmalemma adenosine
triphosphatase from leaf epidermis and mesophyll of Nicotiana rustica L. PI. Physiol.,
Lancaster 63, 936-939.
Membranes from maize root tips
167
MARTIN, H. G. & THORNE, K. J. I. (1974). The involvement of endogenous dolichol in the
formation of lipid-linked precursors of glycoproteins in rat liver. Biochem. J. 138, 281-289.
MOLLENHAUER, H. H. & MOLLENHAUER, B. A. (1978). Changes in the secretory activity of the
Golgi apparatus during the cell cycle in root tips of maize (Zeamays L.). Planta 138, 113-118.
MONTAGUE, M. J. & RAY, P. M. (1977). Phospholipid synthesizing enzymes associated with
Golgi dictyosomes from pea tissue. PL Physiol., Lancaster 59, 225-230.
MORRE, D. J. (1975). Membrane biogenesis. A. Rev. PL Physiol. 26, 441-481.
MORRE, D. J., KARTENBECK, J. & FRANKE, W. W. (1979). Membrane flow and interconversions
among endomembranes. Biochim. biophys. Acta 559, 71-152.
MORRE, D. J. & OVTRACHT, L. (1977). Dynamics of the Golgi apparatus: membrane differentiation and membrane flow. Int. Rev. Cytol. 5 (Suppl.), 61-188.
MORRE, D. J., YUNGHANS, W. N., VIGIL, E. L. & KEENAN, T . W. (1974). Isolation of organelles
and endomembrane components from rat liver: biochemical markers and quantitative
morphometry. In Methodological Developments in Biochemistry, vol. 4 (ed. E. Reid), pp. 195236. New York: Longmans, Green.
NACAHASHI, J. & BEEVERS, L. (1978). Subcellular localization of glycosyl transferases involved
in glycoprotein biosynthesis in the cotyledons of Pisum sativum L. PL Physiol., Lancaster
6i,45i-459NORTHCOTE, D. H. (1971). The Golgi apparatus. Endeavour 30, 26-33.
NORTHCOTE, D. H. (1974). Complex envelope system. Membrane systems of plant cells. Phil.
Trans. R. Soc. B 268, 119-128.
NORTHCOTE, D. H. (1979). The involvement of the Golgi apparatus in the biosynthesis and
secretion of glycoproteins and polysaccharides. Biomembranes 10, 51-76.
ONCUN, A. & MUDD, J. B. (1970). The biosynthesis of steryl glucosides in plants. PL Physiol.,
Lancaster 45, 255-262.
PALMITER, R. D. (1969). Properties of lactose synthetase from mouse mammary gland: role of
a proposed third component. Biochim. biophys. Acta 178, 35-46.
PHILLIPS, R. & BUTCHER, D. N. (1979). Membrane phospholipids of normal and crown gall
callus cultures. Phytochemistry 18, 791-793.
POWELL, J. T. & BREW, K. (1974). Glycosyltransferases in the Golgi membranes of onion stem.
Biochem. J. 142, 203-209.
QUAIL, P. H. (1979). Plant cell fractionation. A. Rev. PL Physiol. 30, 425-484.
QUINN, P. J. & WILLIAMS, W. P. (1978). Plant lipids and their role in membrane function.
Prog. Biophys. molec. Biol. 34, 109-173.
RAY, P. M., EISINCER, W. R. & ROBINSON, D. G. (1976). Organelles involved in cell wall
polysaccharide formation and transport in pea cells. Ber. dt. bot. Ges. 89, 121-146.
RAY, P. M., SHININCER.T. L. & RAY, M. M. (1969). Isolation of /?-glucan synthetase particles
from plant cells and identification with Golgi membranes. Proc. natn. Acad. Set. U.S.A. 64,
605-612.
SHARMA, C. B., BABCZINSKI, P., LEHLE, L. & TANNER, W. (1974). The role of dolicholmono-
phosphate in glycoprotein synthesis in Saccliaromyces cerevisiae. Eur.J. Biochem. 46, 35-41.
SHORE, G. & MACLACHLAN, G. A. (1975). The site of cellulose synthesis. Hormone treatment
alters the intracellular location of alkali-insoluble /?-i,4-glucan (Cellulose) synthetase
activities. J. Cell Biol. 64, 557-571.
SPARACE, S. A. & MOORE, T. S. (1979). Phospholipid metabolism in plant mitochondria.
Submitochondrial sites of synthesis. PL Physiol., Lancaster 63, 963-972.
STUDIER, F. W. (1973). Analysis of bacteriophage T7 early RNAs and proteins on slab gels.
J. molec. Biol. 79, 237-248.
TAUSSKY, H. H. & SHORR, E. (1953). A microcolorimetric method for the determination of
inorganic phosphorus. J. biol. Chem. 202, 675-685.
THOM, M., LAETSCH, W. M. & MARETZKI, A. (1975). Isolation of membranes from sugarcane
cell suspensions: evidence for a plasma membrane enriched fraction. PL Set. Lett. 5,
245-253VEEGER, C , DER VARTANIAN, D. V. & ZEYLEMAKER, W. P. (1969). Succinate dehydrogenase.
Metli. Enzym. 13, 81-90.
WILLIAMSON, F. A., MORRE, D. J. & JAFFE, M. J. (1975). Association of phytochrome with
rough-surfaced endoplasmic reticulum fractions from soybean hypocctyls. PL Physiol.,
Lancaster 56, 738-743.
{Received 20 November 1979)