* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download R - MyCourses
Bisulfite sequencing wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Non-coding DNA wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Molecular cloning wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Butyric acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Protein structure prediction wikipedia , lookup
DNA supercoil wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
CHEM-E4140
Selectivity in Synthesis and Recognition
Lecture 11: Bioactive Compounds
Jan Deska
Laboratory of Organic Chemistry
05.12.2016
structure,
b i n d i n g , polysaccharides
energy
Pri mary B uilding Blocks o f Life
§
the primary metabolism
OH
O
CO2H
pyruvic acid
O
HO
HO
HO
sugars
OH
D-glucose
HO
O
O
–O
SCoA
source of
acetyl
carbon
coenzyme A
(fats,
lipids,
citric
acid
secondary
cycle
metabolites
HO
O
P
O–
OH OH
D-ribose
phosphate
global energy storage
O
ATP
NH
–O
HO2C
CO2H
HO CO2H
citric acid
OH
m u s c u l a r R CO H
2
tissue,
m e s s e n g e r NH2
a m i n e s , . . . L-amino acids
–O
P
O
N
O
O
OH OH
ribonucleotides
O
HO
pyruvic acid
OH
D-glucose
Pri mary B uilding Blocks o f Life
§
HO
the primary metabolism
O
O
–O
HO
SCoA
citric
acid
cycle
HO2C
O
P
O–
OH OH
D-ribose
phosphate
acetyl
coenzyme A
O
ATP
NH
–O
–O
CO2H
HO CO2H
R
P
O
CO2H
NH2
citric acid
controls
N
O
O
O
OH OH
L-amino acids
all chemical reactions
in living things
OH
ribonucleotides
controls
proteins
nucleic acids
structure and catalysis
store genetic informations
N u c l e o b a s e s e n c o d e for Amino a c i d s
Structure o f p r o t e i n s
§
the simple building blocks: proteinogenic amino acids
H2N
H2N
COOH
H2N
COOH
alanine
(Ala, A)
glycine
(Gly, G)
H2N
COOH
valine
(Val, V)
leucine
(Leu, L)
S
H2N
COOH
isoleucine
(Ile, I)
N
H
COOH
proline
(Pro, P)
COOH
H2N
COOH
methionine
(Met, M)
Structure o f p r o t e i n s
§
the simple building blocks: proteinogenic amino acids
OH
H2N
SH
OH
H2N
COOH
serine
(Ser, S)
H2N
COOH
COOH
cysteine
(Cys, C)
threonine
(Thr, T)
HO
HN
H2N
COOH
phenylalanine
(Phe, F)
H2N
COOH
tryptophane
(Trp, W)
H2N
COOH
tyrosine
(Tyr, Y)
Structure o f p r o t e i n s
§
the simple building blocks: proteinogenic amino acids
H2N
NH2
O
COOH
HOOC
O
H2N
H2N
COOH
asparagine
(Asn, N)
COOH
H2N
COOH
H2N
aspartic acid
(Asp, D)
glutamine
(Gln, Q)
H
N
H2N
glutamic acid
(Glu, E)
NH2
H
N
NH
N
H2N
COOH
histidine
(His, H)
H2N
arginine
(Arg, R)
COOH
COOH
H2N
COOH
lysine
(Lys, K)
Structure o f p r o t e i n s
§
primary, secondary and tertiary structures
R
R
R
OH
H2N
N
H
– H2O
O
O
H
N
N
H
O
O
R
R
R
– H2O
§
N
H
O
H
N
N
H
O
R
R
R
O
H
N
N
H
O
R
primary structure depicts a virtual linear alignment
R
O
H
N
N
H
O
R
O
H
N
N
H
O
R
O
Structure o f p r o t e i n s
§
primary, secondary and tertiary structures
§
secondary structure describes 3D-objects formed through H-bonding interactions
Structure o f p r o t e i n s
§
primary, secondary and tertiary structures
§
tertiary structure shows arrangement of subunits to form a complex bulk structure
Inhibition o f Protein Functions
§
proteins as key functional molecules (enzymes and receptors)
§
the vast majority of protein functions crucial for maintaining a living organism
(toxicity vs. pharmaceutical effect)
=
nothing to mess around with, but...
§
pharmaceuticals may:
o
interact with alien proteins (from invading microorganisms, parasites, etc)
o
modulate enzyme activities to a desired level
o
mute receptor answers on demand
1 9 t h C e n t u r y Pharmaceutical Chemistry
CO2H
acetylsalicylic acid
O
O
§
analgetic (pain relief)
§
antipyretic (lowering fever)
§
anti-inflammatory
§
anticoagulant
§
salicylic acid first isolated from willow (Salix) by Löwig, 1838
§
industrial synthesis based on Kolbe process, Bayer 1859
CO2
(5 bar)
ONa
150 °C
O
ONa
OH
1 9 t h C e n t u r y Pharmaceutical Chemistry
CO2H
acetylsalicylic acid
O
O
mode of action
§
selective inhibition of prostaglandine
biosynthesis (via cyclooxygenases)
§
prostaglandins act hormone-like
modulating platellet aggregation,
neuron sensitizing etc
§
acetylation of serine blocks entrance for arachidonic acid
CO2H
CO2H
COX-2
2 x O2
O
O
HOO
arachidonic acid
prostaglandin G2
Modern B lockbusters
Lipitor (Atorvastatin, Pfizer)
OH OH O
O
N
OH
PhHN
Ph
F
§
against athereosclerosis
§
lowering cholesterol blood levels
§
prevents cardiovascular diseases (stroke, cardiac arrest,...)
best-selling drug ever
(>125 billion USD)
Cholesterol biosynthesis
Lipitor (Atorvastatin, Pfizer)
mode of action
§
selective inhibition of mevalonic acid biosynthesis
H
N
R
O
O
H
N
S
OH O
OH O
OH
HO
O
mevalonic acid
3-hydroxy-3-methylglutaryl-coenzyme A
O
P
–O
–O
O
P
–O
–O
O
O
P
–O
O
OH
and
O
P
–O
–O
O
O
P
–O
O
H
H
O
terpenes
H
HO
H
cholesterol
H M G -redu ctase
CoA
S
O
O
NADH
OH O
H3N
HN
H3N
HO
Lys
Ser
NH2
Arg
binding mode for the thioester reduction
§
lower cholerstrol levels in plasma
§
lower risk for plaque
§
lower risk for blocked arteries
Lipitor bound irreversibly inside active site
C e r i v a s t a t i n – a failed competitor
Lipobay (Cerivastatin, Bayer)
OH OH O
N
O
What has happened?
OH
F
§
approval in 1997
§
1998-1999: several deaths related to
Lipobay treatment
§
§
statins often combined with lipid
lowering drugs (fibrates)
§
mutual inhibition of degradation
mechanisms ("statin" + "fibrate")
ü
5-fold increased statin levels
ü
inflammation and degradation of
muscle tissue
ü
kidney failure
2001: withdrawl from market
N u c l e i c a c i d s a s Ta r g e t
–O
–O
P
O
–O
base
O
–O
O
P
O
ribonucleotide
deoxyribonucleotide
O
NH2
N
N
N
N
N
adenine
NH2
cytosine
NH
N
guanine
O
N
N
O
OH H
OH OH
N
base
O
NH2
O
NH
O
N
O
NH
N
O
thymine
uracil
DNA only
RNA only
Base Pairing
thymine/
uracil
R
O
H
N
N
adenine
N
H
H
N
N
O
N
N
N
O
guanine
N
H
cytosine
N
N
N
N
H
N
H
O
N
H
H
R e l a t i o n s h i p DNA - RNA - p r o t e i n s
replication
x2
single nucleotides
§
DNA-targeting drugs can interfere with either the replication or transcription
§
affect in principle all cells (cytostatika)
§
but: applicable fighting against rapidly growing/replicating systems
ü
anti-tumor drugs
ü
anti-virals
Fluorouracil
O
F
NH
N
H
O
§
cytostatic suicide inhibitor
§
on the list of the most important medication in basic
health systems
§
widely applied against various types of cancer
(stomach, breast, colorectal, skin,...)
acts as...
§
so-called anti-metabolite
§
irreversible inhibitor of thymidylate synthase
§
thymineless death of rapidly dividing cells
Fluorouracil
§
transformation of deoxyuracil to deoxythymidine required to provide
DNA building blocks for replication
O
Me+
NH
2–O
3PO
O
N
O
thymidylate
synthase
3PO
Me
NH
Enz
2–O
Enz
O
O–
2–O
S
O
SH
N
O
– Enz
OH
3PO
O
NH
N
O
SH
OH
OH
deoxyuracil monophosphate
§
deoxythymidin monophosphate
deoxyfluorouracil not capable of methylation
Me+
Enz
OPO32–
O–
F
NH
S
O
OH
N
O
Tr o j a n H o r s e s
§
fake nucleotides affect all cells
§
using tumor- or virus-specific activation for selective drug recognition
O
NH
O
N
HO
O
N
NH
N
Aciclovir
anti-herpes
§
NH2
N
HO
O
N
O
NH2
HO
N3
AZT
(azidothymidine)
anti-HIV
manipulated nucleosides show high selectivity as anti-virals
(herpes, HIV, ...)
N
(R)-cytallene
anti-hepatitis
O
Tr o j a n H o r s e s
§
rapid replication of virus-infected cells due to sloppy kinase enzymes
§
kinase-catalyzed phosphorylation renders active DNA-building block
ü
incorporation results in unfunctional DNA
ü
cell death only of the infected cells
O
N
HO
O
N
NH
N
NH2
O
viral
kinase
–O
–O
P
O
N
O
O
N
NH
N
Aciclovir
inactive
active
unfunctional
DNA
NH2
Groove Binders
§
interaction of non-DNA related molecules to the DNA's
grooves renders it unreadable
ü
DNA binders selectively inhibit transscription
§
organometallics as anti-cancer drugs
(cisplatin, carboplatin, oxaliplatin,...)
ü
cross-linking of DNA via purine bases
Cl
NH3
Pt
Cl
NH3
O
O
cisplatin
NH3
Pt
O
NH3
O
carboplatin
Groove Binders
§
interaction of non-DNA related molecules to the DNA's
grooves renders it unreadable
ü
DNA binders selectively inhibit transscription
§
pyrrole amidine antibiotics
§
based on netropsin, isolated from
Streptomyces netropsis (1951)
§
binds selectively to the minor
groove of AT rich DNA
NH
H2N
O
N
N
H
NH
O
Netropsin
O
N
N
H
N
H2N
NH2
Groove Binders
§
interaction of non-DNA related molecules to the DNA's
grooves renders it unreadable
ü
DNA binders selectively inhibit transscription
§
pyrrole amidine antibiotics
§
modulation of the pyrrole pattern
allows for selective interactions
with particular nucleobases
O
N
N
N
H
O
HN
NH
N
O
N
O
N
HN
NH3+
HN
CO2
–
O
H
N
O
N+
N
N
H
N
NH
O
N
O
N
H
N
O
in clincal trials against prostate cancer
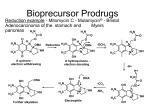
Selectivity in Transport
§
selective transport of a drug to the desired organ crucial for effect
§
unfavorable polarity, rapid degradation etc. pose problems
ü
masking of the pharmaceutical motif = pro-drugs
OH
HO
H
N
OH
H
N
H
N
HO
adrenaline
ephedrine
amphetamine
highly polar
highly unpolar
peripheral effect
acts in brain
hypertensive effect
intoxicative effect
Selectivity in Drug D e l i v e r y
§
selective transport of a drug to the desired organ crucial for effect
§
unfavorable polarity, rapid degradation etc. pose problems
ü
masking of the pharmaceutical motif = pro-drugs
O
N
O
H2N
O
O
N
NH
N
O
NH2
O
C13H27
Cl
Val-Aciclovir
slow hydrolytic
release
O2N
HN
Cl
O
Chloramphenicol palmitate
selective enzymatic hydrolysis
in dudenal guts